Category: Parkinson's Disease: Neuroimaging
Objective: To investigate whether changes in the lumbar paravertebral muscles (LPM) can be detected using muscle ultrasonography (USG) in patients with Parkinson’s disease (PD, PwP) suffering from camptocormia (CC).
Background: Camptocormia is defined as an abnormal flexion of the spine by more than 30 degrees, which can be seen in PD [1]. While CC-related muscle edema and atrophy in the LPM has been shown in several radiological and pathological studies [2], the value of USG in the demonstration of these changes is yet to be understood.
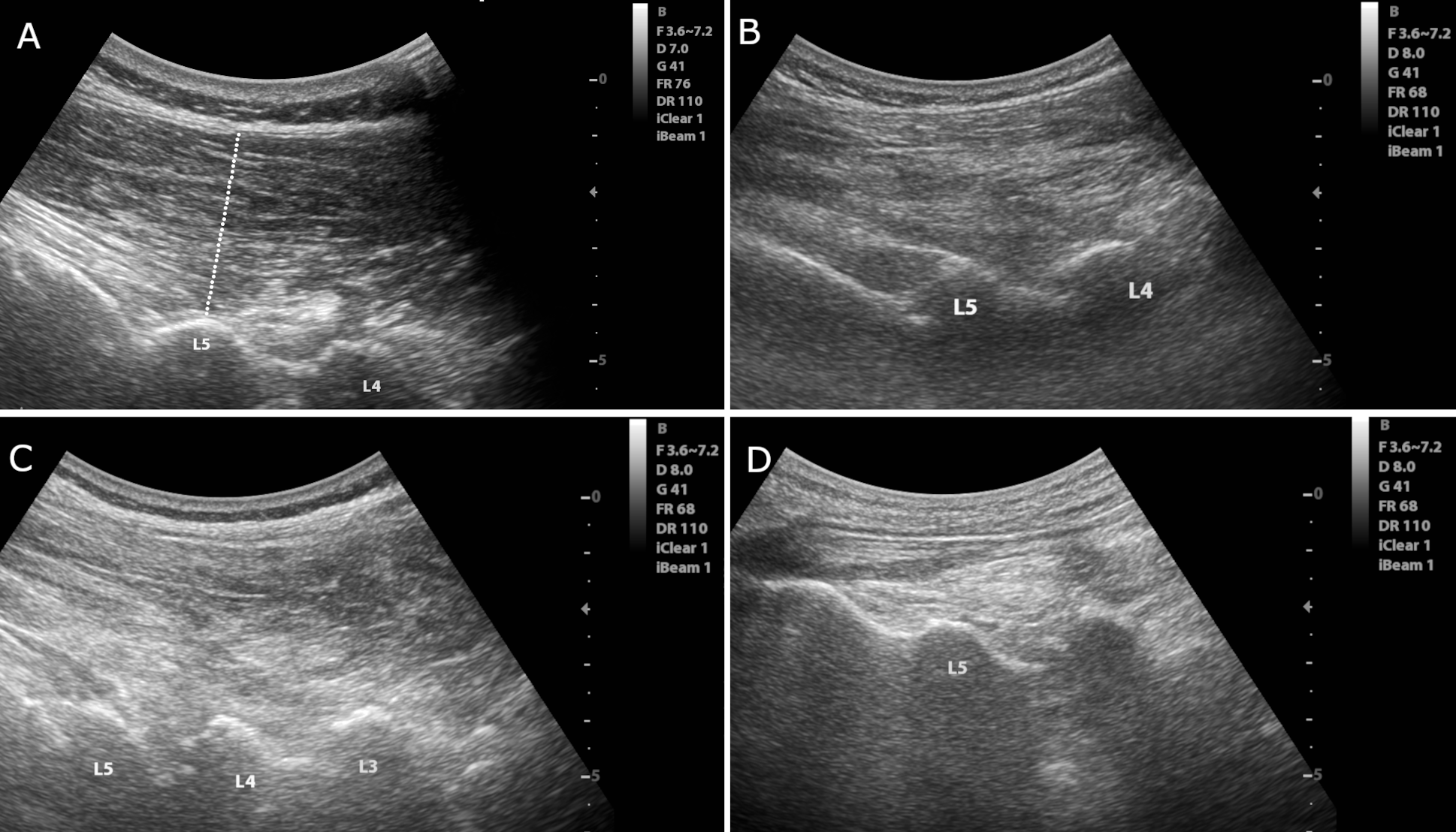
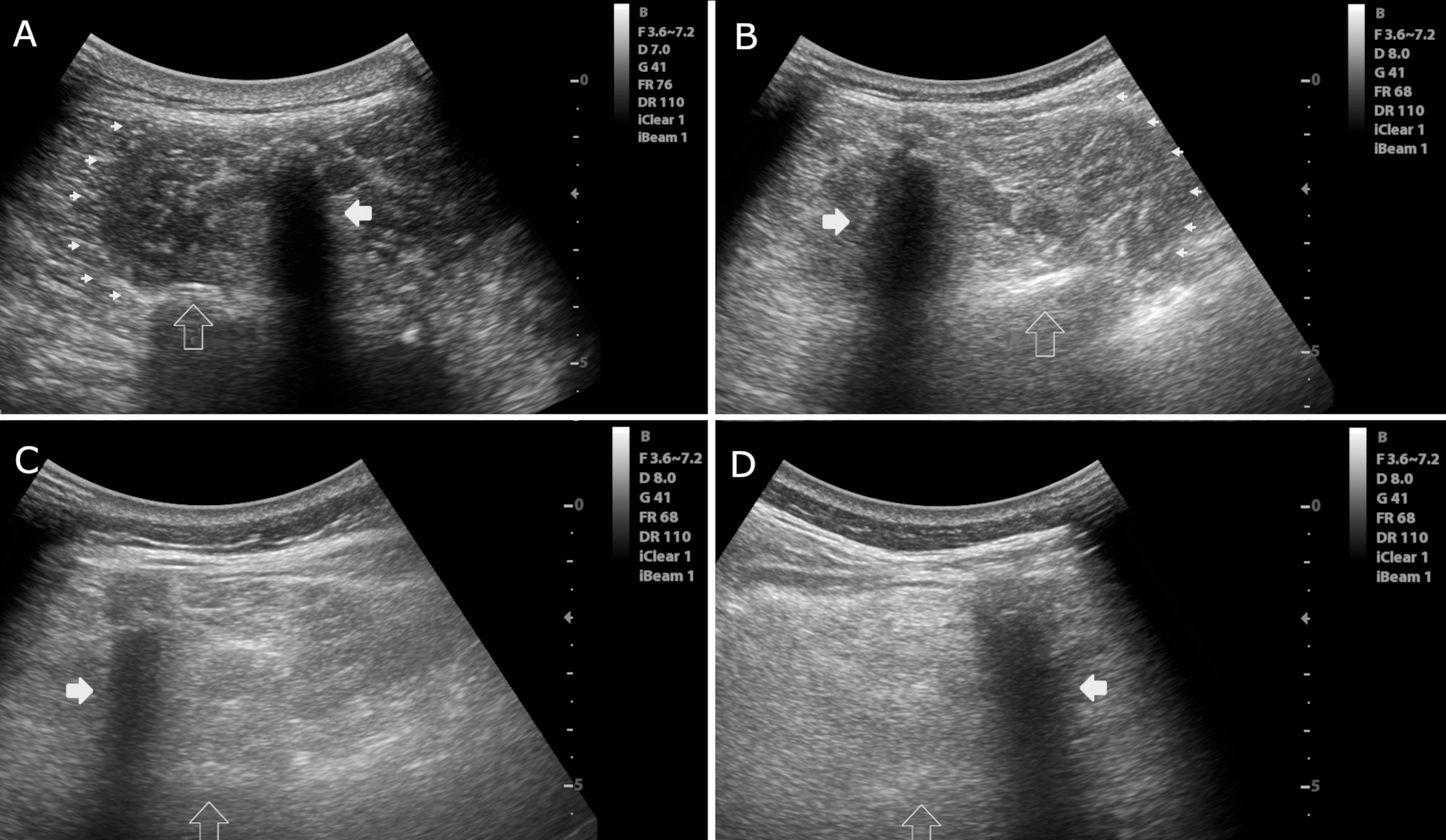
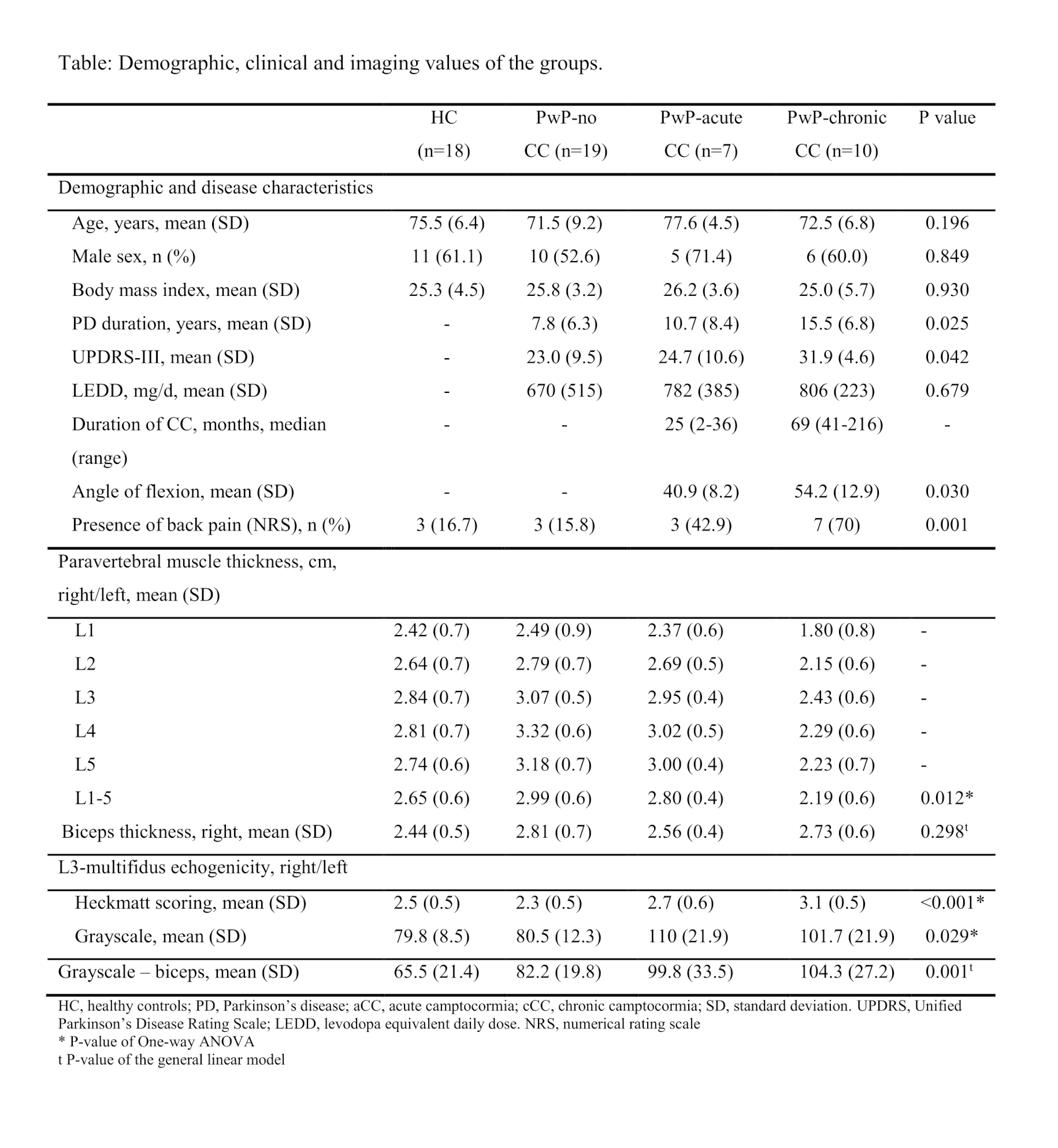
Method: Data on muscle thickness and echogenicity from the LPM collected by two blinded raters using muscle USG in groups of seven PwP with acute CC, 10 PwP with chronic CC, 19 PwP without CC, and 18 healthy controls (HC) were compared. The thickness of the LPM between L1-L5 was assessed by linear measurements [Figure-1]. The echogenicity of the LPM at the level of L3 was assessed using the Heckmatt scale and quantitative grayscale analysis [Figure-2]. Reliability in assessments was evaluated using intra-class correlations (ICC). The groups were compared using a univariate general linear model with age, sex, BMI, and biceps thickness as covariates.
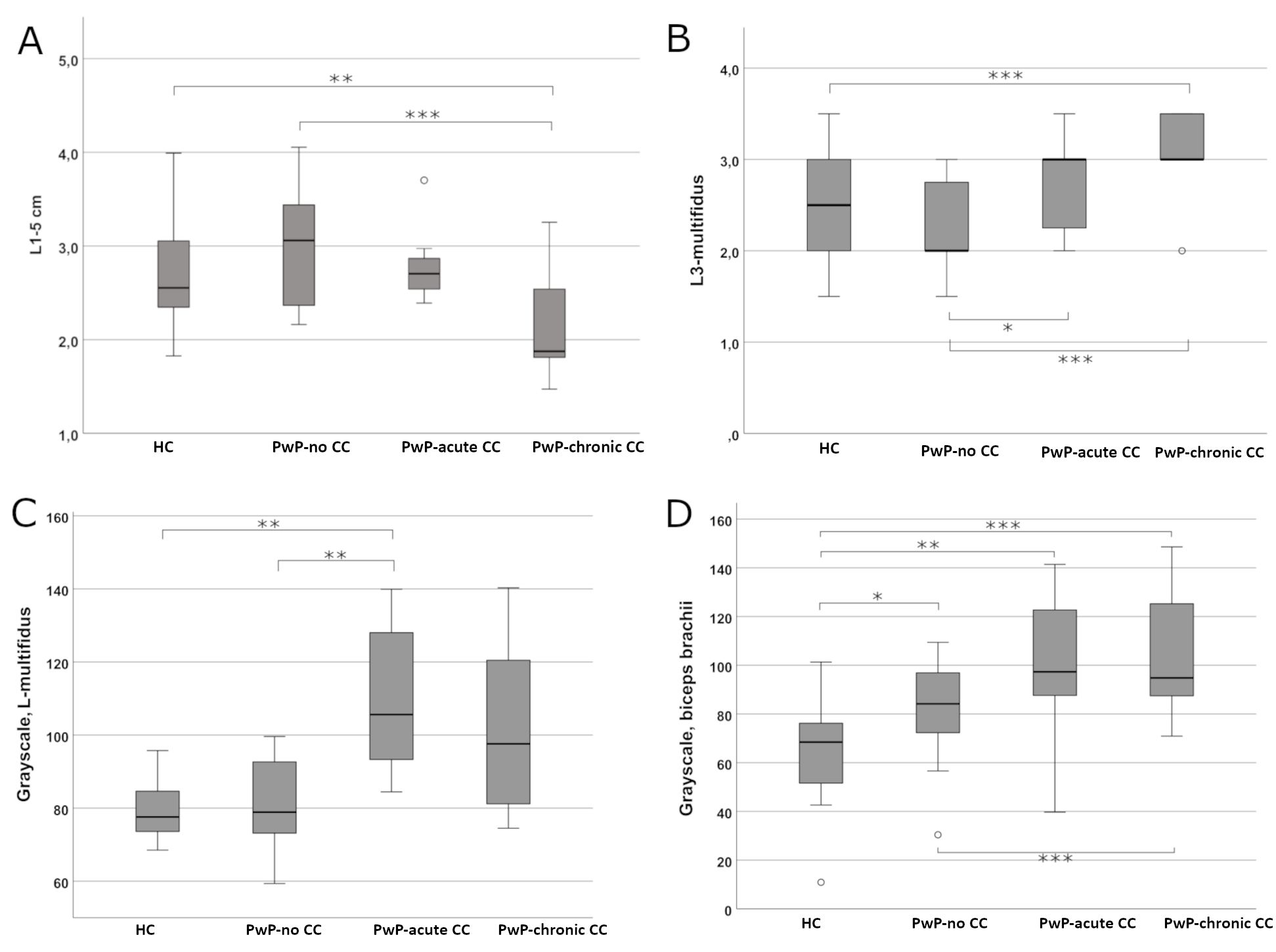
Results: The ICC showed substantial reliability for all assessments. The model showed that PwP with chronic CC had a significantly thinner LPM compared to PwP with no CC (p<0.01) and HC (p<0.001). The difference between PwP with chronic and acute CC was significant at the margin (p=0.05). The echogenicity Heckmatt scores were higher in the acute and chronic CC groups of PwP than in PwP with no CC (p<0.05). PwP with chronic CC also had higher Heckmatt scores than HC (p<0.01). Quantitative grayscale analysis also showed a higher muscle echogenicity in PwP with acute CC compared to PwP with no CC or HC. Additionally, the echogenicity of the biceps was also higher in all PwP than in HC [Table, Figure-3].
Conclusion: Muscle USG is a reliable tool for the assessment of the LPM in PwP with CC. In addition, CC-associated changes in the LPM may be detected using muscle USG, which is a practical and inexpensive imaging tool.
References: [1] Margraf NG, Granert O, Hampel J, Wrede A, Schulz-Schaeffer WJ, Deuschl G. Clinical Definition of Camptocormia in Parkinson’s Disease. Mov Disord Clin Pract 2017;4:349–57.
[2] Margraf NG, Wrede A, Deuschl G, Schulz-Schaeffer WJ. Pathophysiological concepts and treatment of camptocormia. J Parkinsons Dis 2016;6:485–501.
To cite this abstract in AMA style:
R. Yilmaz, R. Wolke, N. Puls, M. Sorgun, G. Deuschl, D. Berg, N. Margraf. Detection of Camptocormia Using Ultrasound in Parkinson’s Disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/detection-of-camptocormia-using-ultrasound-in-parkinsons-disease/. Accessed January 8, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/detection-of-camptocormia-using-ultrasound-in-parkinsons-disease/