Category: Parkinson’s Disease: Clinical Trials
Objective: To assess the efficacy of BIA 28-6156 in delaying clinically meaningful motor progression in PD patients who have a PD-risk associated variant in the GBA1 gene. Safety and tolerability of BIA 28-6156 also will be investigated.
Background: Heterozygous GBA1 pathogenic variants are the most common genetic risk factor for PD, accounting for 5-15% of PD patients. On average, GBA-PD patients present a clinically distinct course with an earlier age of onset and higher rate of disease progression with faster cognitive impairment as compared with idiopathic PD patients [1]. Data from previous studies with BIA 28-6156, an allosteric activator of the enzyme GCase, in GBA-PD patients are consistent with GCase activation [2].
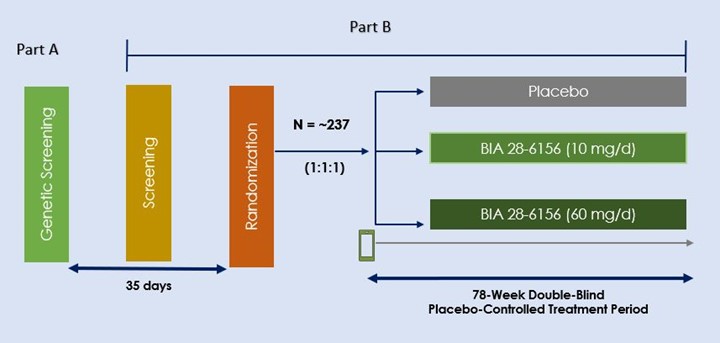
Method: Phase 2, multicenter, randomized, double-blind (DB), placebo-controlled study, with Part A (genetic screening) and Part B (DB treatment up to 78 weeks). Approximately 237 genetically confirmed GBA-PD adult patients will be randomized in a 1:1:1 allocation ratio to BIA 28-6156 10 mg/day, BIA 28-6156 60 mg/day, or matching placebo. Clinical diagnosis of PD for at least 1 to 7 years, a modified H&Y score ≤2.5, and a MoCA score ≥22 are required for inclusion. Patients must be receiving a stable dose of PD medication for at least 30 days before screening (Part B) and will continue receiving usual PD medications throughout the study. Patients with a LRRK2 pathogenic variant will not be eligible to participate.
Results: Primary endpoint is time from baseline to first clinically meaningful progression on motor aspects of experiences of daily living, as assessed by ≥2-points increase in MDS-UPDRS Part II and no improvement (≥0-points) in MDS-UPDRS Part III. Secondary endpoints include safety, tolerability, time from baseline to first increase of ≥5-points in MDS-UPDRS Part III, worsening on the CGI-C, PGI-C and first LEDD increase. Change from baseline to Week 78 in the MDS-UPDRS Parts I-IV, modified H&Y scale, PD-CRS score, PDQ-39 and EQ-5D-5L scores will be investigated.
Conclusion: This time-to-event study will evaluate the efficacy and safety of a once-daily GCase activator, BIA 28-6156, as a disease-modifying therapy for GBA-PD patients.
References: [1] Smith L.; Schapira, A.H.V., Cells. 2022;11,1261.
[2] ADPD, Abstract 524A, A 28-day dosing trial of the glucocerobridase allosteric activator LTI-291 in GBA-PD.
To cite this abstract in AMA style:
L. Magalhães, M. Fonseca, G. Castilla-Fernández, R. Costa, D. Simon, J. Holenz, N. Mendonça. Design of a time-to-event study in GBA Parkinson’s Disease patients: Efficacy and safety of BIA 28-6156, an allosteric activator of beta-glucocerobridase (GCase) [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/design-of-a-time-to-event-study-in-gba-parkinsons-disease-patients-efficacy-and-safety-of-bia-28-6156-an-allosteric-activator-of-beta-glucocerobridase-gcase/. Accessed October 22, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/design-of-a-time-to-event-study-in-gba-parkinsons-disease-patients-efficacy-and-safety-of-bia-28-6156-an-allosteric-activator-of-beta-glucocerobridase-gcase/