Category: Huntington's Disease
Objective: This study aims to estimate the longitudinal minimal clinically important difference (MCID) for HD-relevant, cognitive, motor, and functional outcome measures. We used the new evidence-based HD Integrated Staging System (HD-ISS) designed to provide a research framework for characterizing the HD spectrum to categorize the participants.
Background: Patient-reported outcome (PRO) measures are often used to detect any changes while evaluating the effectiveness of a given treatment or intervention, but statistically significant changes in health-related quality of life PRO scores within a clinical trial may not necessarily signify clinical relevance.[1] MCID represents the smallest within-person change on an outcome measure that is considered meaningful to the patient.[2] Anchor-based MCID methods evaluate the relationship between changes in an outcome measure and patient-reported clinical importance of that change.[3]
Method: We analyzed data from Enroll, a longitudinal, observational study of HD (N =10,598) using the HD-ISS system to define progression groups during timeframes ranging from 12 to 36 months. The anchor was the Physical Component Score (PCS) [4] of the PRO 12-item short-form health survey (SF-12), and HD-relevant, motor, cognitive and functional outcome measures were used as independent, external criterion outcome measures. Multiple, independent, linear mixed effect regression models were used to calculate MCID for each external criterion by HD-ISS staging group.
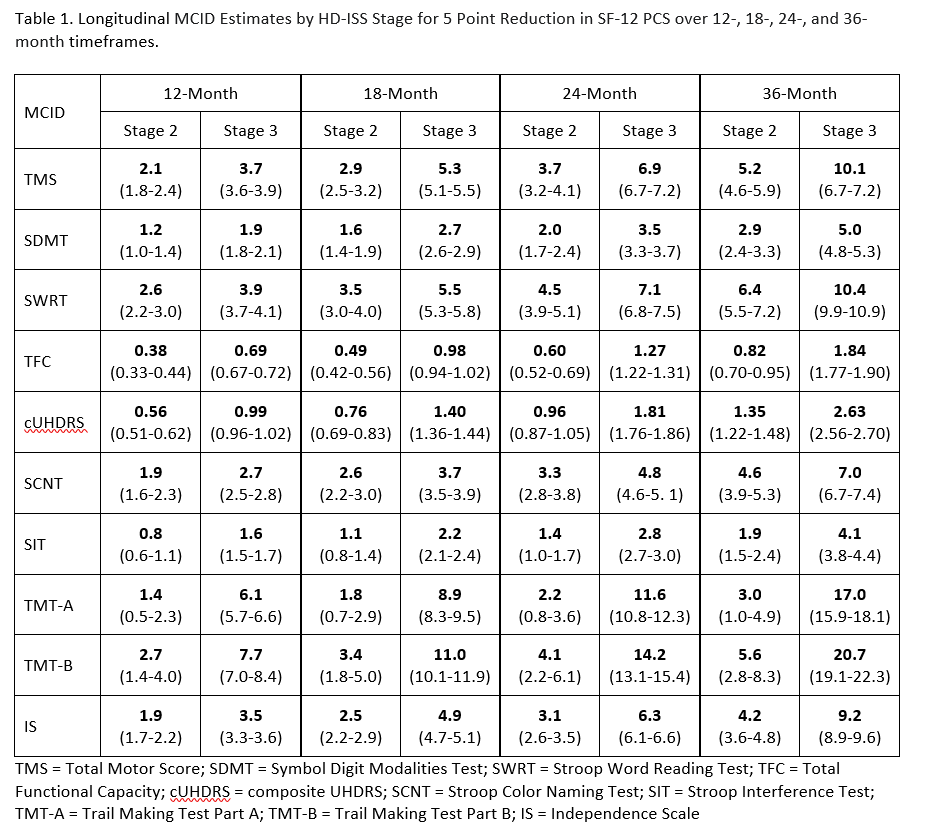
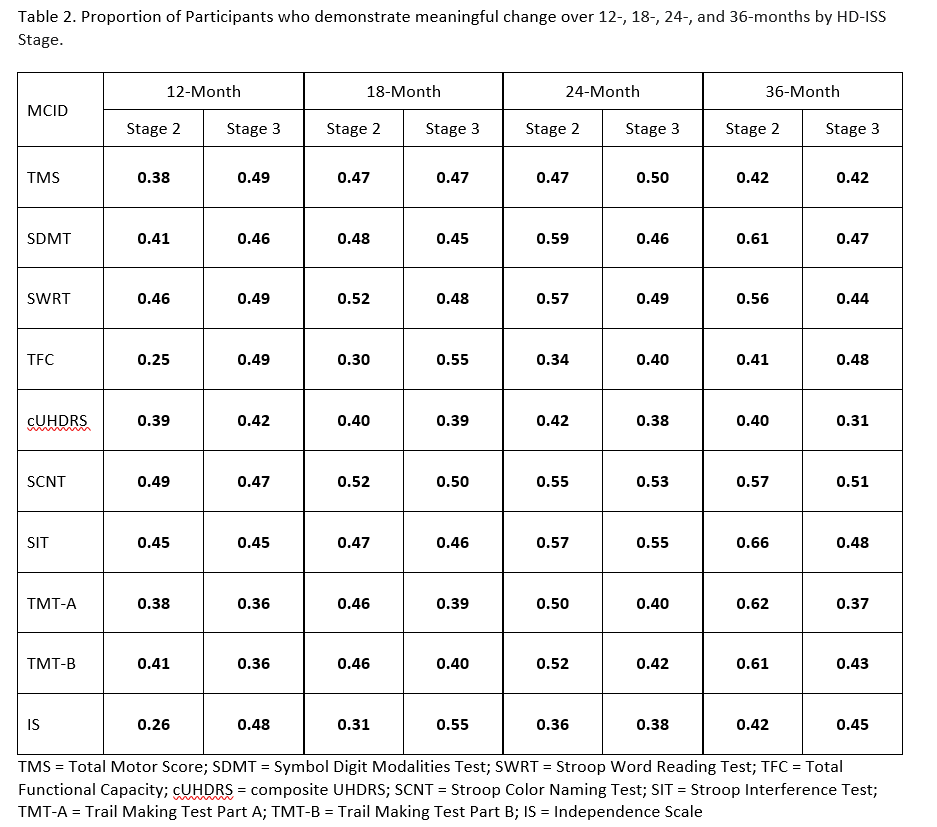
Results: MCID estimates varied by progression stage. MCID estimates increased as stage progression increased and as the timeframe increased. The combination of Stage 3 and 36-month timeframe had the largest estimates, whereas the combination of Stage 2 and 12-months had the smallest. [Table 1] Generally, higher proportions of Stage 2 than Stage 3 participants experience meaningful change on the cognitive measures over 24 months. [Table 2]
Conclusion: This is the first study to our knowledge that examined MCID estimation thresholds for HD. The results can be used to improve clinical interpretation of study outcomes and inform trial design.
References: 1. U.S. Food and Drug Administration. (2009) Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims.
Retrieved from https://www.fda.gov/media/77832/download.
2. Page P. (2014) Beyond statistical significance: clinical interpretation of rehabilitation research literature. Int J Sports Phys Ther;9(5):726–36.
3. Jaeshke R, Singer J, Guyatt G. (1989) Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials;10:407– 15.
4. Ware Jr, John E., Mark Kosinski, and Susan D. Keller (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity.Medical
care 34.3: 220-233.
To cite this abstract in AMA style:
J. Hamilton, J. Mills, G. Stebbins, J. Long, R. Fuller, S. Sathe, M. Roche, C. Sampaio. Defining minimal clinically important differences in Huntington’s disease: An anchor-based approach [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/defining-minimal-clinically-important-differences-in-huntingtons-disease-an-anchor-based-approach/. Accessed April 21, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/defining-minimal-clinically-important-differences-in-huntingtons-disease-an-anchor-based-approach/