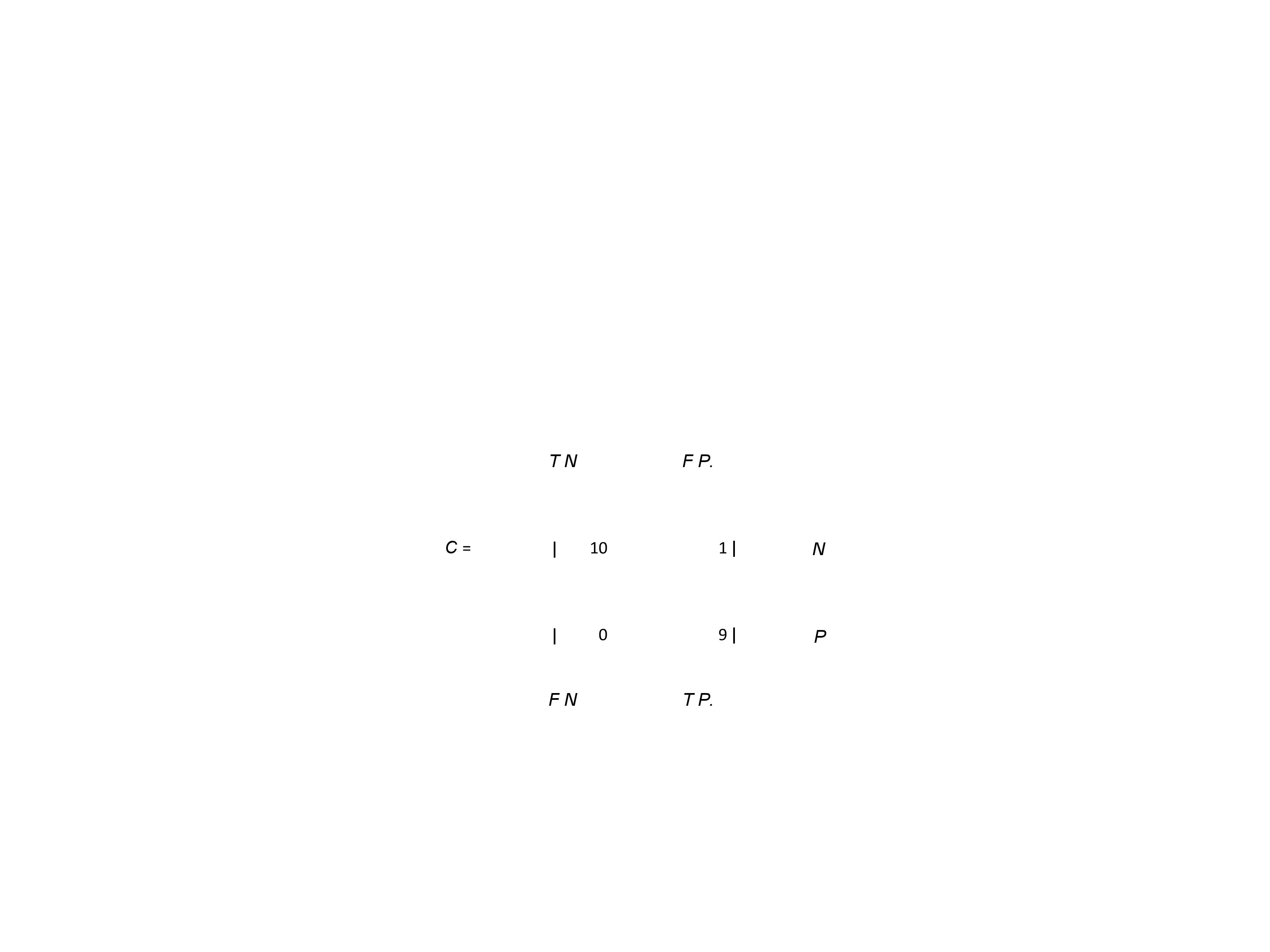Session Information
Date: Saturday, October 6, 2018
Session Title: Surgical Therapy: Parkinson's Disease
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To render the significance of microelectrode recording and elucidate its prognostic role in response to STNDBS.
Background: Studies[1]-[9] have correlated local field potential(LFP) and multi unit activity(MUA) features with DBS enhancement. Yet, quantification and prediction of the subject specific movement had not done.
Methods: The followed methods and techniques are used in this study: Data acquisition, data processing and feature selection, classification and regression.
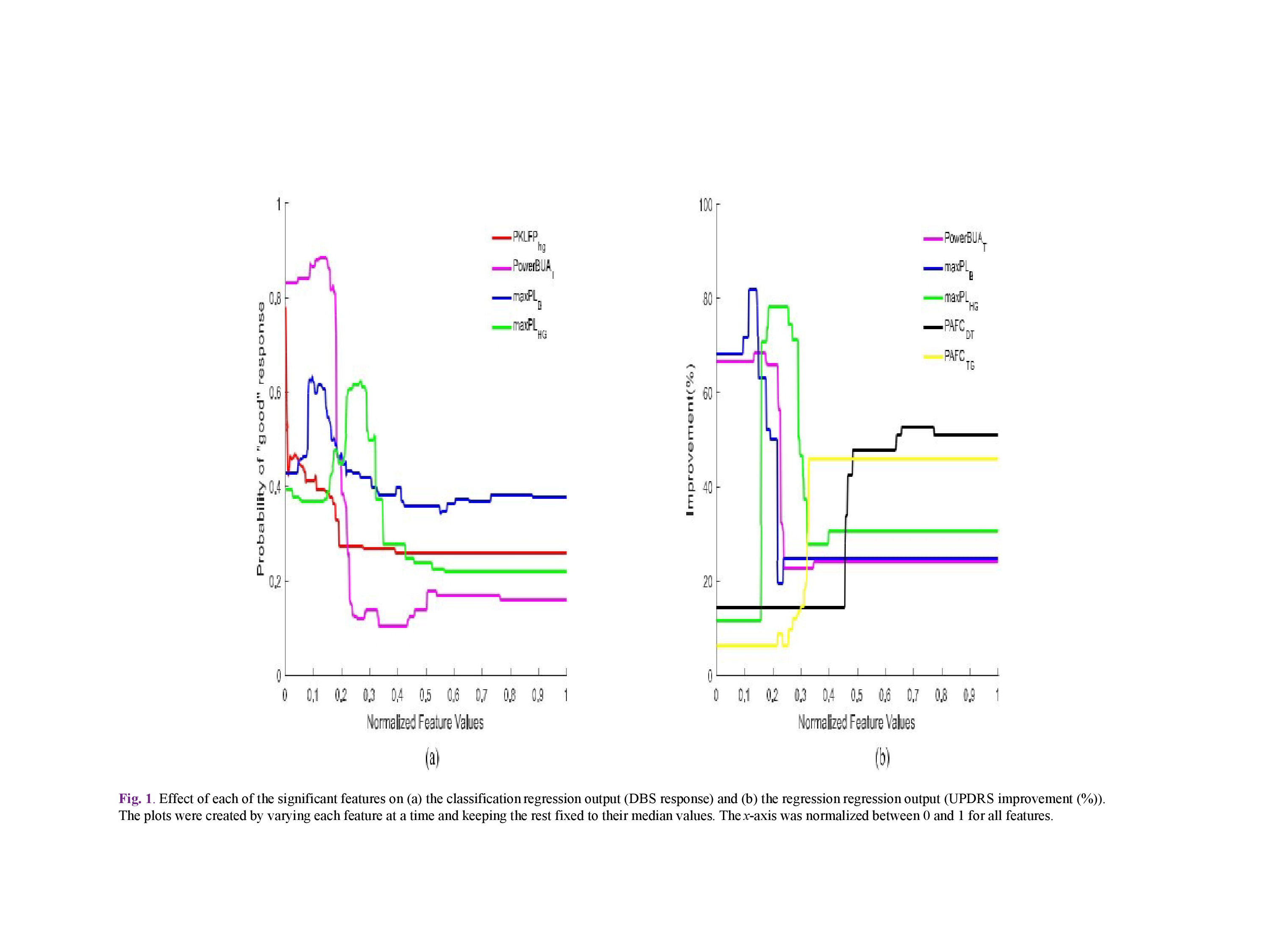
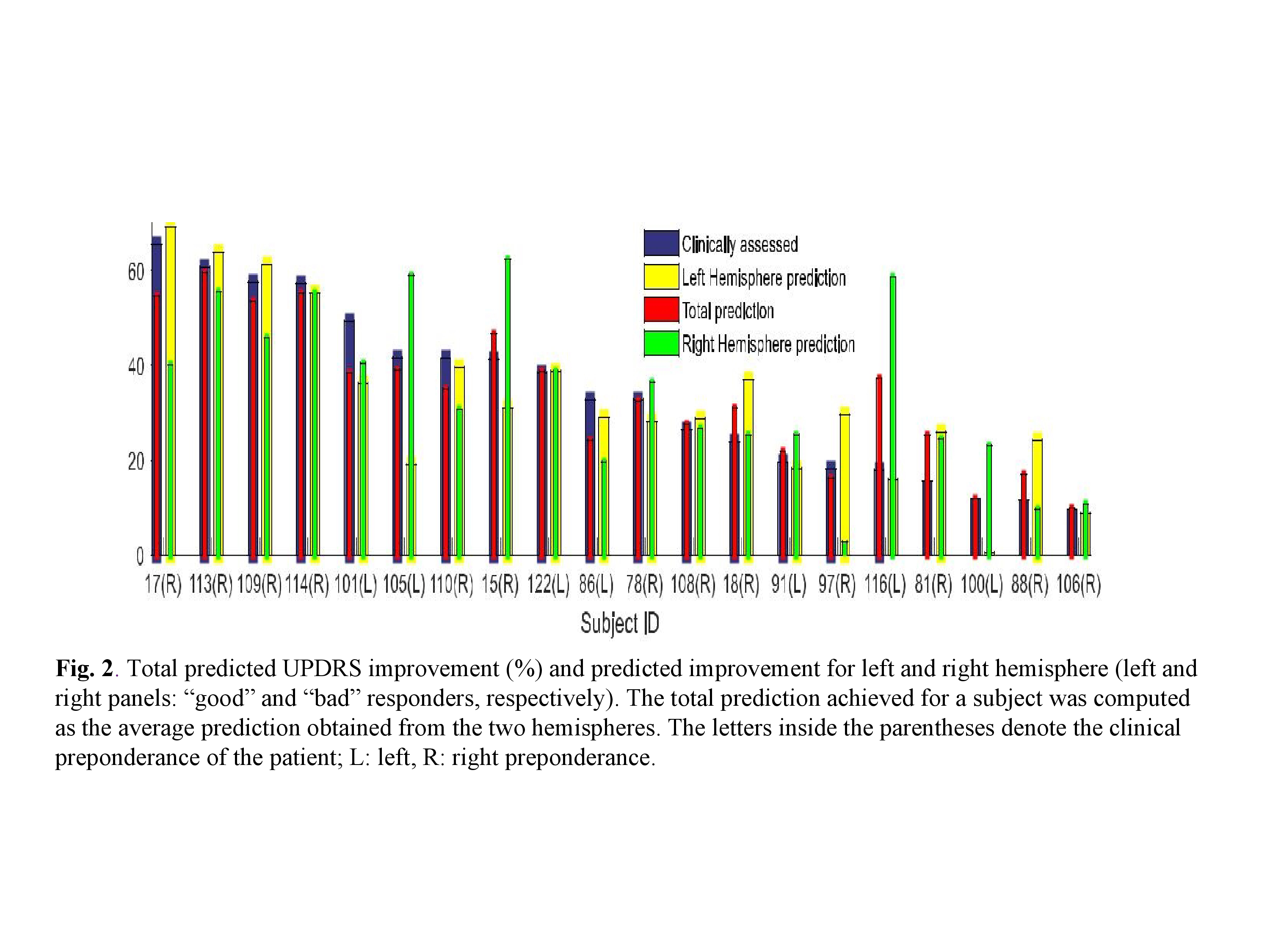
Results: STN-DBS response with prediction We applied backward elimination feature selection scheme [10],[11] and found that four features attained a maximum MCC value of 0.9045, with a confusion matrix C of the form (TABLE I), where, N = TN + FP is the total number of actual “poor” responders (11 in our case) and P = FN + TP is the total number of actual “good” responders (9 in our case). In other words, only one “poor” responder was classified falsely in the “good” STN-DBS response group. The most significant features were found to be PKLFPHG, PowerBUAT, maxPLB, and maxPLHG (TABLE II) with FIs 0.1495, 0.9142, 0.3899, and 0.5982. The effect of each feature on model response is shown(Fig 1a). There is a negative correlation between the values of the above mentioned features and the probability of “good” response, e.g., when PKLFPHG, maxPLB, and maxPLHG attain their median values, an increase in the value of PowerBUAT leads to a decrease of “good” response probability. In order to extract MER features that can quantitatively predict the improvement in the “off”-state UPDRS scale pre and postoperatively, backward elimination scheme used. Five features achieved a maximum correlation co efficient of 0.9178, corresponding to an NMSE of 3.38%; PowerBUAT, maxPLB, maxPLHG, PAFCDT, and PAFCTG (TABLE II) with FIs 0.2058, 0.0561, 0.1506, 0.0764, and 0.1669, respectively. Clinically assessed and OOB predicted UP-DRS improvement (%) for all patients is shown in Fig2. The effect of each feature on the model response can be seen in Fig 1 (b). PowerBUAT, maxPLB, and maxPLHG were negatively correlated with the UPDRS improvement, while PAFCDT and PAFCTG were positively correlated with the latter.
Conclusions: Proposed approach can employ a small number of signal features of STN-neurons to forecast, separately for each patient, the behavioral outcome of STN DBS justifying further investigation and possibly clinical applications.
References: 1. A. L. Benabid, “Deep brain stimulation for Parkinson’s disease,” Curr. Opin. Neurobiol., vol. 13, no. 6, pp. 696–706, 2003. 2. A. L. Benabid et al., “Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease,” Stereotact. Funct. Neurosurg., vol. 62, no. 1–4, pp. 76–84, 1994. 3. Giladi et al., “Validation of the freezing of gait questionnaire in patients with Parkinson’s disease,” Mov Disord, vol. 24, no. 5, Pp: 655–661, 2009. 4. Yashar Sarbaz1, et.al., “A review of presented mathematical models in Parkinson’s disease: black‑ and gray‑box models”, Med Biol Eng Comput, (2016) 54:855–868. 5. A. A. Kuhn et al., “Pathological synchronization in the subthalamic nu-cleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity,” Exp. Neurol., vol. 215, no. 2, pp. 380–387, 2009. 6. S. Little et al., “Beta band stability over time correlates with Parkinsonian rigidity and bradykinesia,” Exp. Neurol., vol. 236, no. 2, pp. 383–388, 2012. 7. A. A. Kuhn et al., “Reduction in subthalamic 8-35 Hz oscillatory activ-ity correlates with clinical improvement in Parkinson’s disease,” Eur. J. Neurosci., vol. 23, no. 7, pp. 1956–1960, 2006. 8.C. C. Chen et al., “Complexity of subthalamic 13-35 Hz oscillatory activity directly correlates with clinical impairment in patients with Parkinson’s disease,” Exp. Neurol., vol. 224, no. 1, pp. 234–240, 2010. 9. A. Zaidel et al., “Subthalamic span of β oscillations predicts deep brain stimulation efficacy for patients with Parkinson’s disease,” Brain, vol. 133, no. 7, pp. 2007–2021, 2010. 10. K. P. Michmizos et al., “Prediction of the timing and the rhythm of the Parkinsonian subthalamic nucleus neural spikes using the local field po-tentials,” IEEE Trans. Inf. Technol. Biomed., vol. 16, no. 2, pp. 190–197, Jun. 2011. 11. K. Kostoglou et al., “Prediction of the Parkinsonian subthalamic nucleus spike activity from local field potentials using nonlinear dynamic models,” in proc. IEEE 12th Int. Bioinfo. Bioeng. (BIBE), pp. 298–302, 2012.
To cite this abstract in AMA style:
V. Rama Raju, R. Borgohain. Deep brain stimulation and its efficacy using microelectrode recording [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/deep-brain-stimulation-and-its-efficacy-using-microelectrode-recording/. Accessed April 20, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/deep-brain-stimulation-and-its-efficacy-using-microelectrode-recording/