Category: Parkinson's Disease: Pathophysiology
Objective: The objective is to characterise parkinsonian phenotypes in genetic and pharmacological models of Parkinson’s disease (PD) using zebrafish.
Background: Parkinson’s disease (PD) is a progressive nervous system disorder that affects key functions in 1% of the population aged above 60 years old. The neuropathological alterations that underlie PD include the loss of dopaminergic neurons and abnormal intraneuronal accumulation of Lewy bodies. While the pathogenesis of PD remains unclear, there is evidence that various processes might contribute to it. Currently, there is no cure available for PD patients, other than drugs to alleviate symptoms.
Here, the zebrafish is used to generate models of PD and to analyse the behavioural defects associated with disease. The small size and transparent nature of zebrafish renders it an excellent model for non-invasive circuit analysis, enabling detailed study and link of neuronal circuits with behaviour. Zebrafish are amenable to a wide range of genetic tools allowing for studies that link physiology to genetic perturbations. Furthermore, starting at larval stages, zebrafish exhibit a large collection of behaviours which can be quantified in a high-throughput manner and serve as a powerful screening tool.
Method: The pharmacological model used is based on the incubation with the neurotoxicant chemical MPTP, which is widely known chemical to kill dopaminergic cells in zebrafish and cause hypolocomotion. Additionally, several genetic models of PD have been generated using CRISPR/Cas9 to target genes associated with the disease, such as park2, park7, lrrk2 and pink1. A transgenic line overexpressing the human α-synuclein gene has also been generated.
Using these distinct PD models, a deep characterisation of locomotor patterns is conducted using a high-throughput behavioural assay to record swimming events of individual fish at a high frequency over large timescale. Kinematic parameters are analysed in three manoeuvres: fast acoustovestibular escape responses, slow forward swims and routine turns.
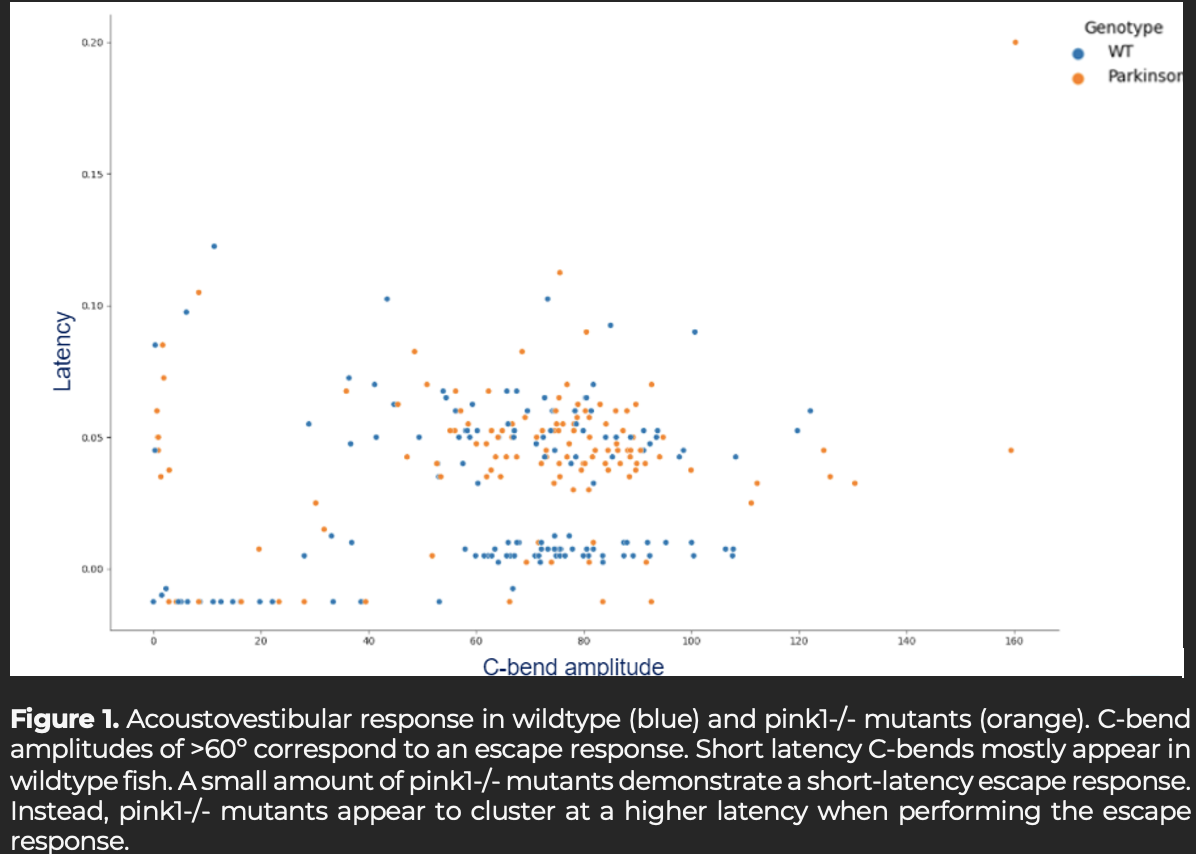
Results: In response to an acoustovestibular stimulus, PD mutants demonstrate long-latency escape responses, which could be regarded as a delayed response [figure1]. Additionally, PD mutants exhibit less swimming higher tail beat amplitude compared to control.
Conclusion: Altogether, by building a phenotypic profile of PD could enable screening of candidate chemicals for PD in zebrafish.
To cite this abstract in AMA style:
TM. Tzotzolaki, J. Garcia Fernandez, F. de Santis, J. Terriente. Deep behavioural analysis of zebrafish models of Parkinson’s disease [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/deep-behavioural-analysis-of-zebrafish-models-of-parkinsons-disease/. Accessed May 9, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/deep-behavioural-analysis-of-zebrafish-models-of-parkinsons-disease/