Category: Parkinson’s Disease: Clinical Trials
Objective: Characterize and compare the time-course of Parkinson’s disease (PD) symptoms following 24h/day continuous subcutaneous infusion (CSCI) of foslevodopa/foscarbidopa and oral levodopa/carbidopa (LD/CD).
Background: As PD progresses, symptoms are no longer well controlled by oral medication due to a narrowing therapeutic window. Foslevodopa/foscarbidopa is a new soluble formulation of carbidopa and levodopa prodrugs designed to maintain stable levodopa exposure for 24h/day delivery via CSCI.
Method: Individually optimized therapeutic doses of foslevodopa/foscarbidopa were delivered as CSCI for 28 days in advanced PD patients (Study M15-739, NCT03374917). The time‑course of “Off” time, “On” time and “On time with non-troublesome dyskinesia”, were assessed via PD diaries from baseline (BL, before switching from oral LD/CD to foslevodopa/foscarbidopa) through study end (Day 28). In addition to comparison of the averaged daily diary assessments, a generalized additive mixed effects model was used to assess the difference between the treatments throughout the day.
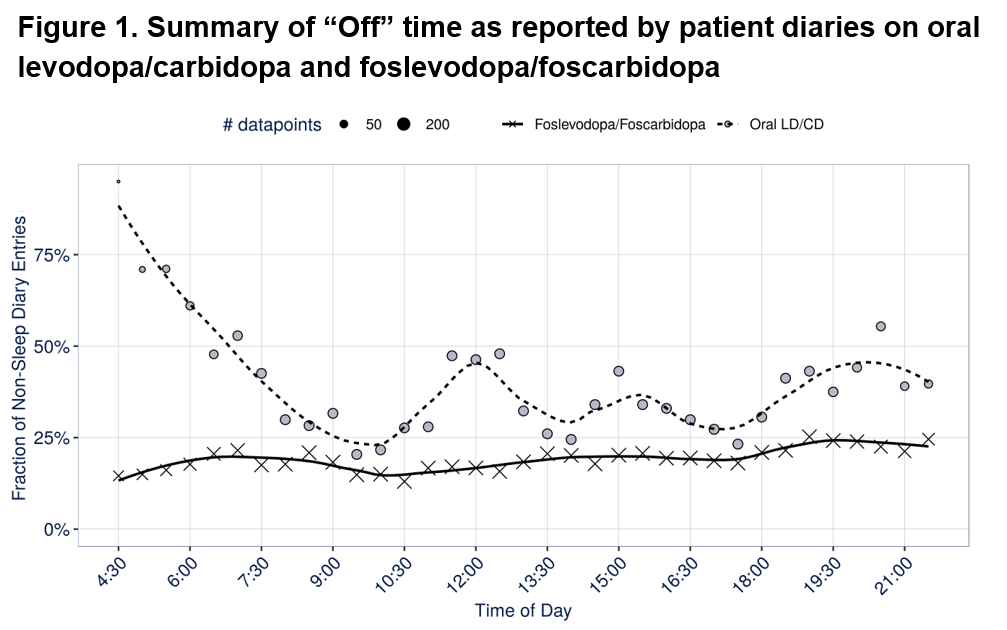
Results: Twenty patients with data available both on oral LD/CD and on foslevodopa/foscarbidopa were included in this analysis. Overall, in this analysis, receiving foslevodopa/foscarbidopa was associated with a significant reduction in the probability to be in the “Off” state (Odds ratio [95% confidence interval] 0.407 [0.236 – 0.702], p < 0.01) and a significant increase in the probability to be in the “On” state without dyskinesia (Odds ratio [95% CI] 2.75 [1.08 – 6.99], p < 0.05). Considering the time-course of the symptoms over the day, the symptoms with foslevodopa/foscarbidopa CSCI were relatively evenly distributed throughout the day, whereas fluctuation was observed while on oral LD/CD (Figure 1).
Conclusion: Compared to oral LD/CD, foslevodopa/foscarbidopa decreased “Off” time while increasing “On” time without dyskinesia, reducing fluctuations over the day. The amount of dyskinesias, both troublesome and non-troublesome was also reduced and more stable over the day. This suggests that achieving a stable pharmacokinetic (PK) profile with foslevodopa/foscarbidopa throughout the day reduces motor fluctuations.
To cite this abstract in AMA style:
S. Stodtmann, M. Rosebraugh, W. Robieson, M. Facheris. Daily Time-Course of Efficacy of Continuous Subcutaneous Infusion of Foslevodopa/foscarbidopa in Advanced Parkinson’s Disease Patients from a Phase 1b Study [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/daily-time-course-of-efficacy-of-continuous-subcutaneous-infusion-of-foslevodopa-foscarbidopa-in-advanced-parkinsons-disease-patients-from-a-phase-1b-study/. Accessed January 8, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/daily-time-course-of-efficacy-of-continuous-subcutaneous-infusion-of-foslevodopa-foscarbidopa-in-advanced-parkinsons-disease-patients-from-a-phase-1b-study/