Category: Rare Genetic and Metabolic Diseases
Objective: D-penicillamine (DPA) belongs to the most frequently used drugs in Wilson’s disease (WD) treatment. Despite the effectiveness in WD treatment, DPA could be involved in many adverse drug reactions (ADRs), which should be recognized early.
Background: WD is a genetic disorder with copper accumulation in tissues leading to clinical symptoms (mainly hepatic and neuropsychiatric). The pharmacological treatment of WD is based on drugs leading to negative copper body balance – chelators (DPA, trientine), and zinc salts.
Method: We present a patient with ocular myasthenia gravis (MG) induced with DPA.
Results: 51-year-old man was diagnosed with WD in March 2021 by differential diagnosis of liver disease. Treatment with DPA was introduced, up to 1000mg. After 15 months of treatment, diplopia and evening ptosis occurred. The neurological examination was normal apart from the ocular defects. Brain magnetic resonance and routine blood tests including thyroid functions were normal. Repetitive nerve stimulation and single fiber electromyography did not reveal abnormal neuromuscular junction transmission. However, the edrophonium test was positive, and high level of antibodies against acetylcholine receptors (AChR ab) in serum was found (15,3nmol/l, N<0.45). Computed tomography of mediastinum did not find thymoma. The diagnosis of MG, probably induced by DPA was established. DPA was stopped, zinc salts 180mg/day as anti-copper agents and pyridostigmine 240mg/day were introduced. Diplopia and a ptosis subsided in few days. During follow up visit after six months, patient did not present the MG symptoms. The AchR ab level remained elevated (6,3nmol/l). The pyridostigmine was stopped, the neurological symptoms did not reoccur till now (February 2023).
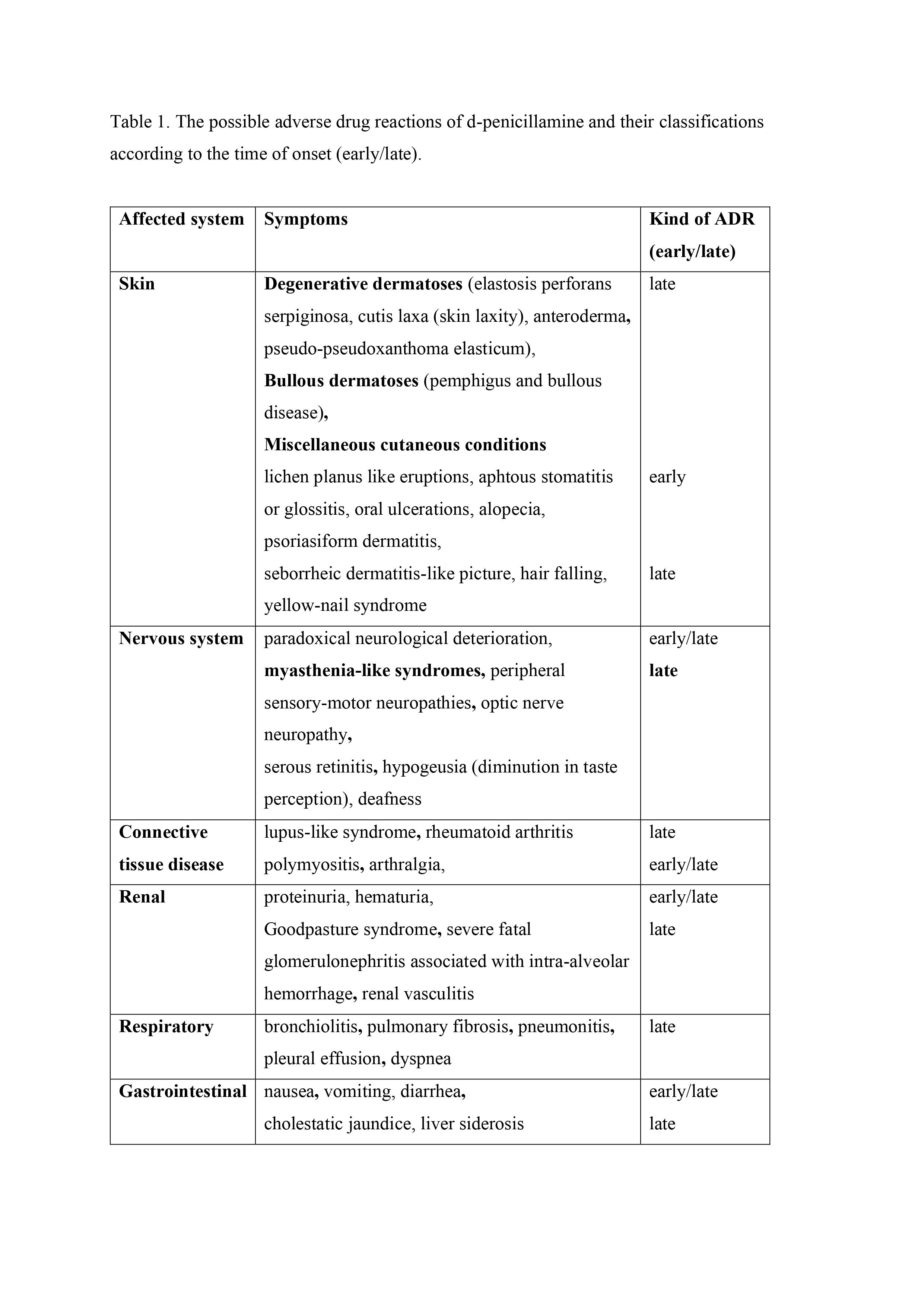
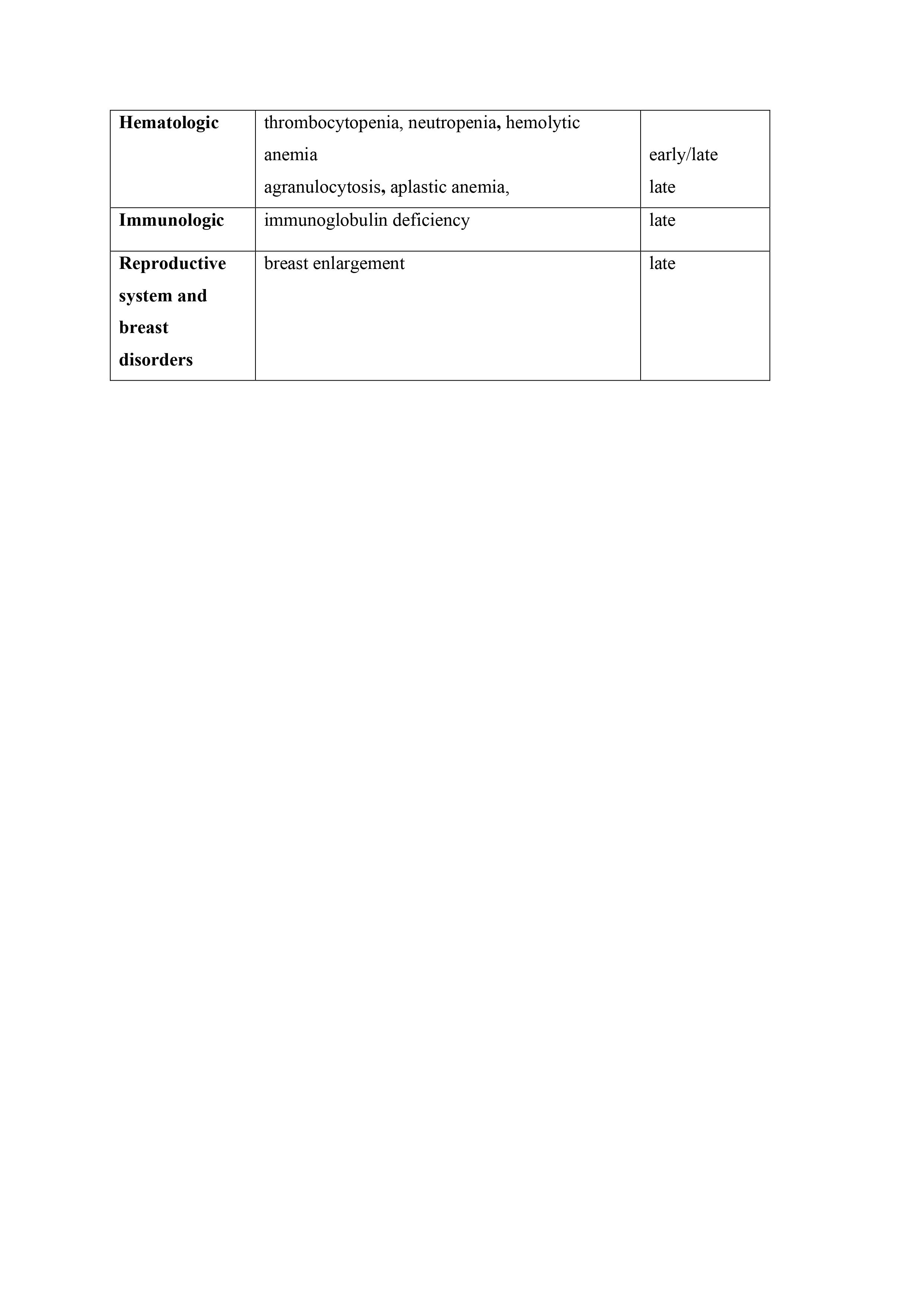
Conclusion: MG belongs to the rare and usually reversible complications of treatment with DPA. It occurs usually 2-12 month after treatment initiation. Symptoms are usually ocular, with good response to pyridostigmine and remits within 1 year after DPA treatment cessation. Sometimes, it could persist, and DPA treatment is only a trigger of MG occurrence. The long-term observation after DPA cessation, AChR ab disappearance, as well as lack of clinical symptoms let us only retrospectively establish the MG etiology in such cases. As DPA could induce several ADRs, the knowledge about them should be obligatory for physicians involved in WD management (Tab.1).
To cite this abstract in AMA style:
A. Antos, T. Litwin, J. Bembenek, M. Skowrońska, I. Kurkowska-Jastrzębska, A. Członkowska. D-penicillamine induced myasthenia gravis – the possible complication of Wilson’s disease treatment. [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/d-penicillamine-induced-myasthenia-gravis-the-possible-complication-of-wilsons-disease-treatment/. Accessed December 21, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/d-penicillamine-induced-myasthenia-gravis-the-possible-complication-of-wilsons-disease-treatment/