Category: Parkinson’s Disease: Clinical Trials
Objective: A meta-analysis of PGIC results was performed using combined data from 3 different studies of CVT-301 in patients with PD over a total of 52 weeks.
Background: CVT-301 is approved for treatment of OFF episodes in PD patients on oral carbidopa/levodopa. In the phase 3, placebo-controlled, double-blind study, CVT-301 improved motor function in patients experiencing OFF periods at week 12, measured by lower Unified Parkinson Disease Rating Scale Part III (UPDRS-III) scores at 30 minutes postdose. 58% achieved and maintained an ON state<60 minutes postdose vs 36% on placebo. 71.4% of patients on CVT-301 reported improvement in PGIC vs 46.4% on placebo. In a 12-month, open-label extension study, 66.7%-91.9% patients reported PGIC improvement. Also, in a 12-month, open-label, observational control (OC) safety study >75% reported improvement at 3, 6 and 12 months.
Method: PGIC results from the double blind and open-label studies of CVT-301 were combined. Patients on oral dopa-decarboxylase inhibitor/levodopa, experiencing >2 hours/day of OFF were randomized to CVT-301(60 mg/84 mg) or placebo or OC (no study drug administered), to be taken ≤5 times/day on return of OFF symptoms.
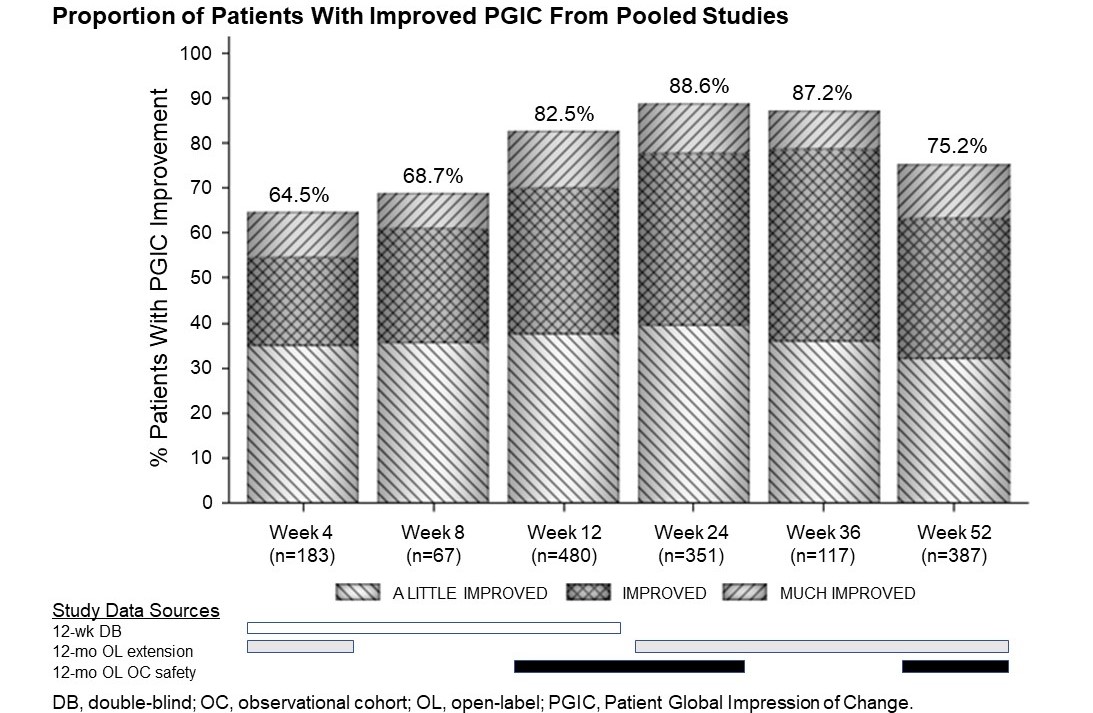
Results: Pooled results (84 mg dose) show that the proportion of patients taking CVT-301 in
controlled and open-label studies who reported PGIC improvement (“a little improved, “improved”, or “much improved”) ranged from 64.5%-88.6% from 4-52 weeks, respectively [Figure]. Of those reporting any improvement, patients with “improved” and “much improved” increased from 29.5% at week 4 to 51.3% at week 36 and 43.2% at week 52. Additional meta-analyses will be presented.
Conclusion: Proportion of patients on CVT-301 (84 mg) reporting improvement in their PD on the PGIC increased over the study periods of 4-52 weeks. PGIC improvement, in addition to improvements measured by UPDRS-III support the efficacy of CVT-301 in PD patients.
To cite this abstract in AMA style:
M. Saint-Hilaire, A. Corbin, P. Zhao, D. Kegler-Ebo. CVT-301 (levodopa inhalation powder) improves Patient Global Impression of Change (PGIC) over 1 year in patients with Parkinson’s disease (PD): a meta-analysis [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/cvt-301-levodopa-inhalation-powder-improves-patient-global-impression-of-change-pgic-over-1-year-in-patients-with-parkinsons-disease-pd-a-meta-analysis/. Accessed January 2, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/cvt-301-levodopa-inhalation-powder-improves-patient-global-impression-of-change-pgic-over-1-year-in-patients-with-parkinsons-disease-pd-a-meta-analysis/