Category: Parkinson’s Disease: Clinical Trials
Objective: To assess the correlation between sleep and quality of life (QoL) in people with Parkinson’s disease (PwP) receiving either oral levodopa/carbidopa immediate-release (LD/CD-IR) tablets or continuous subcutaneous infusion (CSCI) of foslevodopa/foscarbidopa (LDp/CDp) for 12 weeks.
Background: Sleep disorders are prevalent and cause significant burden in PwP. The PD Sleep Scale-2 (PDSS-2), PD Questionnaire 39-items (PDQ-39), and Movement Disorder Society-Unified PD Rating Scale (MDS-UPDRS Part II) measure sleep, QoL, and activities of daily living (ADL) in PwP, respectively. A 12-week double-blind active-controlled phase 3 trial (NCT04380142) showed clinically meaningful improvements in motor fluctuations, sleep, and QoL in LDp/CDp-treated PwP vs baseline (BL), with the sleep and QoL improvements unable to claim statistical significance vs oral due to the trial’s hierarchical testing design.1
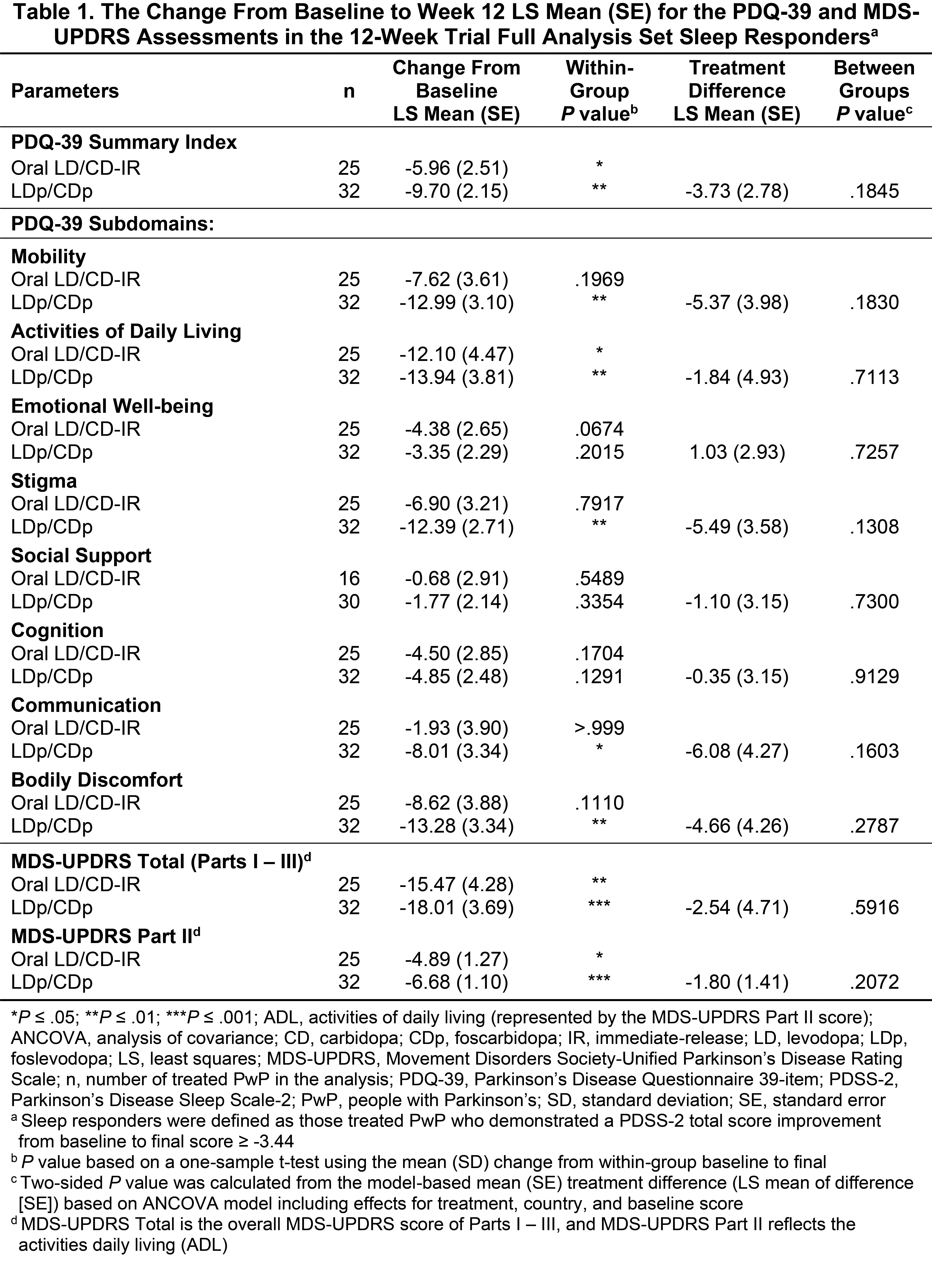
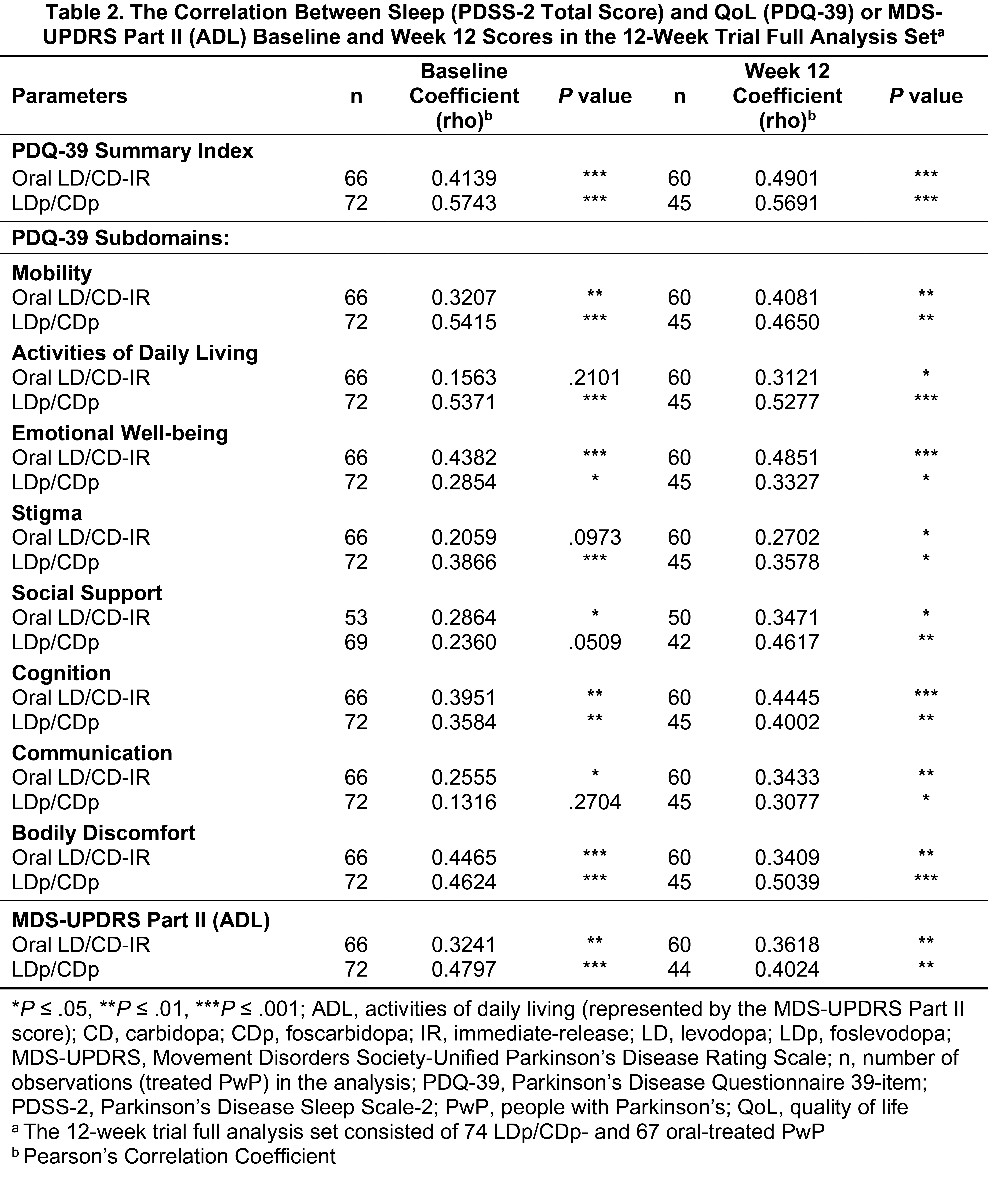
Method: This post hoc study analyzed the number of sleep responders (PwP demonstrating clinically meaningful PDSS-2 total score [TS] improvement of ≥-3.442 change from BL to week 12 [wk-12]) in the LDp/CDp vs oral LD/CD-IR treatment arms from the above 12-week phase 3 trial. Sleep responders LS mean (SE) PDQ-39 and ADL changes from BL were compared within and between arms. Pearson’s correlation coefficient was calculated in the full analysis set. All reported P values were nominal with no multiplicity corrections.
Results: The 12-week trial full analysis set had significantly more sleep responders in the LDp/CDp (n=32/44 with existing data, P<.01, 73%) vs oral (n=25/59 with existing data, 42%) arms. Table 1 lists sleep responder PDQ-39/ADL changes from BL, with no significant differences seen between arms. A significant moderate positive correlation3 between PDSS-2 TS and PDQ-39 Summary Index was seen in all LDp/CDp-treated PwP at BL and wk-12 (Table 2, Figure 1), with Table 2 listing additional consistently positive correlation data. The 12-week trial safety profile showed LDp/CDp CSCI was well tolerated and generally safe.1
Conclusion: The LDp/CDp arm had significantly more sleep responders vs the oral arm, and sleep responders demonstrated significant QoL improvements from BL in both groups. No confounding correlations were observed and significant weak to moderate positive correlations between sleep and QoL/ADL were seen in the full analysis set in both arms.
Table 1.
Table 2.
Figure 1.
References: 1. Soileau MJ, et al. Lancet Neuro. 2022;21:1099–1109.
2. Horvath K, et al. Parkinsons Dis. 2015;2015:970534.
3. Mukaka MM. Malawi Med J. 2012;24:69-71.
To cite this abstract in AMA style:
I. Malaty, O. Vaou, R. Hauser, B. Bergmans, P. Odin, M. Shah, L. Bergmann, L. Harmer, R. Gupta, K. Chaudhuri. Correlation Between Sleep and Quality of Life in People With Parkinson’s Disease Treated With Continuous Subcutaneous Infusion of Foslevodopa/Foscarbidopa [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/correlation-between-sleep-and-quality-of-life-in-people-with-parkinsons-disease-treated-with-continuous-subcutaneous-infusion-of-foslevodopa-foscarbidopa/. Accessed January 8, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/correlation-between-sleep-and-quality-of-life-in-people-with-parkinsons-disease-treated-with-continuous-subcutaneous-infusion-of-foslevodopa-foscarbidopa/