Session Information
Date: Thursday, June 23, 2016
Session Title: Parkinson's Disease: Clinical Trials II and Non-PD Clinical Trials
Session Time: 12:00pm-1:30pm
Objective: To evaluate the copper (Cu) chelation efficacy of orally administered D-penicillamine loaded nanoparticles to that of conventional D-penicillamine therapy for 90 days in Wistar rat model for non-Wilsonian brain Cu toxicosis.
Background: Oral D-penicillamine therapy is unable to attenuate the brain Cu overload and neurological manifestations in Wilsons disease patients.
Methods: Atomic absorption spectrophotometry, high performance liquid chromatography, neurobehavioral and histopathological studies, and nanoparticles preparation/physico-chemical characterization were carried out.
Results: D-penicillamine nanoparticles exhibited mean 274.09 nm size and less than 29.32% of D penicillamine release under in vitro conditions. Pharmacokinetics studies showed augmented levels of D-penicillamine in brain of nanoparticles based D-penicillamine delivery group compared to conventional Dpenicillamine delivery group. Conventional and nanoparticles based D-penicillamine therapy resulted in significantly improved neuromuscular coordination and memory along with concomitant increase in urinary Cu levels in Wistar rat model of copper toxicosis. Conventional D-penicillamine therapy resulted in negative rhodanine staining of brain and liver sections corroborated by 16.4% and 60.1% reduction in brain and hepatic Cu content, respectively compared to non-treated Cu-intoxicated group. However, liver and brain sections of nanoparticles based D-penicillamine therapy group demonstrated grade 1 Cu and no Cu depositions substantiated by 47.2% and 32.8% reduction in hepatic and brain Cu content, respectively in comparison to non-treated Cu-intoxicated group 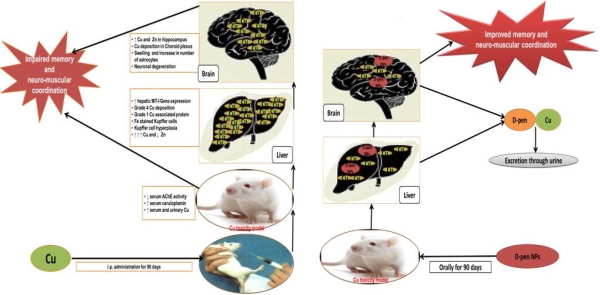 .
.
Conclusions: Our data demonstrates the first in vivo evidence for therapeutic efficacy of D-penicillamine nanoparticles in chelating more brain Cu and mitigating neurological deficits even at half the dose as given in conventional D-penicillamine therapy.
ACBICON 2015, PGIMER, Chandigarh, India.
To cite this abstract in AMA style:
A. Pal, B.R. Thapa, R.K. Vasishta, R. Prasad. Copper chelation efficacy of alginate/chitosan based D-penicillamine nanoparticles in rat model of non-Wilsonian brain copper toxicosis [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/copper-chelation-efficacy-of-alginatechitosan-based-d-penicillamine-nanoparticles-in-rat-model-of-non-wilsonian-brain-copper-toxicosis/. Accessed October 17, 2025.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/copper-chelation-efficacy-of-alginatechitosan-based-d-penicillamine-nanoparticles-in-rat-model-of-non-wilsonian-brain-copper-toxicosis/
