Category: Rating Scales
Objective: To outline the process of conceptualizing the prototype for the International Parkinson and Movement Disorder Society Parkinson’s Disease Psychosis Rating Scale (MDS-PDPRS).
Background: Approximately 50% of Parkinson’s disease (PD) patients experience psychosis, which significantly impacts patient outcomes and caregiver burden (1). Currently, there is no validated Clinical Outcome Assessment (COA) specifically for assessing the functional impact of PD psychosis (PDP). Developing a COA for PDP will aid in understanding its effects, tracking changes over time, and evaluating therapeutic interventions in clinical trials.
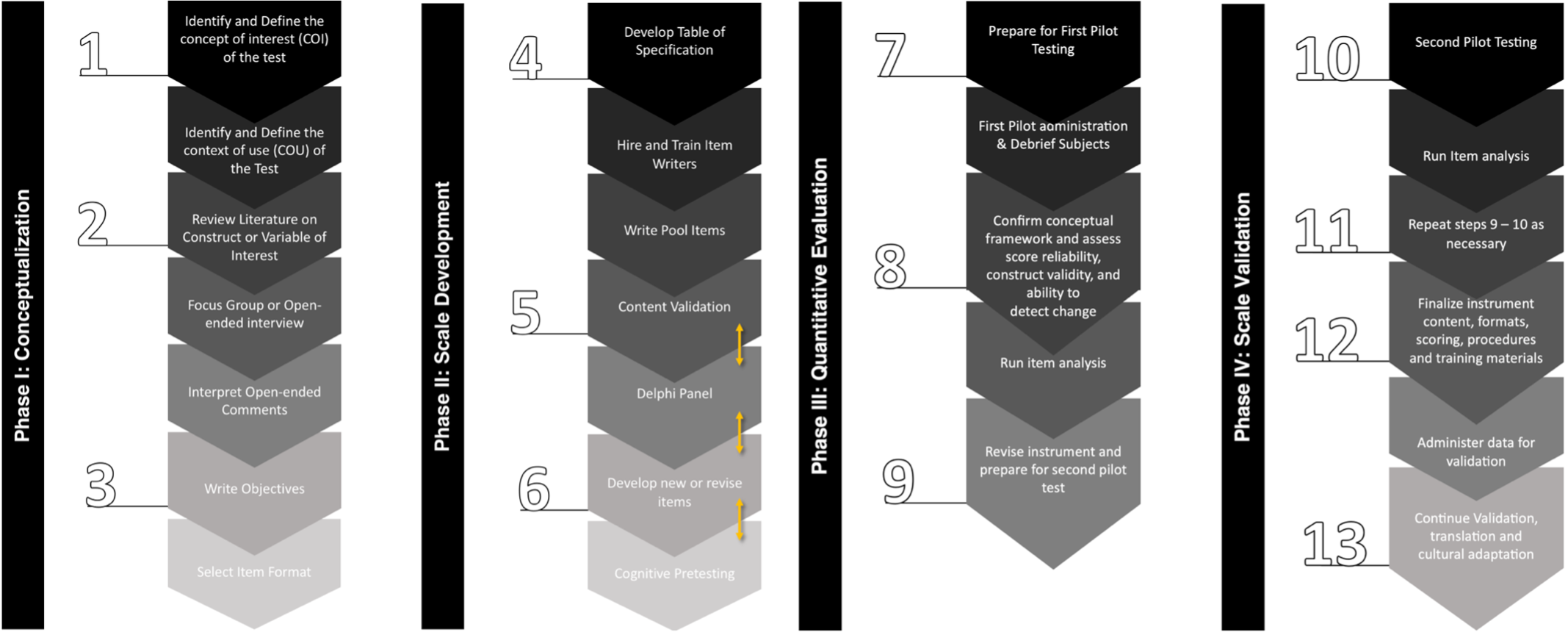
Method: A mixed-methods approach was employed, adhering to best practices for developing a patient-focused COA for clinical trials (2-4). Four of the 13 steps outlined in Figure 1 were implemented, involving research centers in North America, Africa, Europe, and Oceania with native English speakers. Data triangulation from a scoping review (5), eight focus groups composed of PD patients and care partners, and seven Delphi discussions among eleven experts (including neurologists, psychiatrists, and PD care partners) were utilized to establish the concept of interest (COI), the context of use (COU), items, and response options for the initial MDS-PDPRS prototype.
Results: The MDS-PDPRS was conceptualized as a Clinician-Reported Outcome Assessment (ClinRO) designed to evaluate the severity of PDP and its impact on daily functioning following the International Classification of Functioning, Disability, and Health (ICF) framework. It is specifically tailored for PD and should not be used if psychosis symptoms can be attributed to other causes of Parkinsonism. The initial MDS-PDPRS prototype comprises a five-item Diagnostic Screening Questionnaire to establish PDP and then, for those fulfilling entry criteria, a two-part inventory of 14 Likert-type questions: Part 1: PDP Severity (six items) and Part 2: Functional Impact (eight items).
Conclusion: The conceptualization of the MDS-PDPRS was completed, and the prototype is now ready for cognitive pretesting and content validation.
Acknowledgments: The MDS-PDPRS program is supported by a grant from Acadia Pharmaceuticals to the International Parkinson and Movement Disorders Society
Scale development and validation process
References: References:
1. Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord. 2009;24(15):2175-86.
2. US Food and Drug Administration. Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims [Internet]. 2009 [cited 2021 Aug 10]. p. 1–39.
3. Boateng GO, Neilands TB, Frongillo EA, Melgar-Quiñonez HR, Young SL. Best Practices for Developing and Validating Scales for Health, Social, and Behavioral Research: A Primer. Front Public Health. 2018;6:149.
4. Sampaio C, Goetz CG, Schrag A. Rating Scales in Parkinson’s Disease: clinical practice and research. New York: Oxford University Press; 2012. 342 p.
5. Mangone G, Tosin MHS, Goetz CG, Stebbins GT, Mestre TA. Unveiling Assessment Gaps in Parkinson’s Disease Psychosis: A Scoping Review. Mov Disord. 2024. Epub ahead of print. PMID: 38291860.
To cite this abstract in AMA style:
M. Tosin, T. Mestre, G. Stebbins, G. Mangone, S. Videmsky, S. Ali, D. Aarsland, J. Goldman, T. Khoo, S. Lewis, P. Martinez-Martin, O. Ojo, J. Pagonabarraga, A. Schrag, D. Weintraub, C. Goetz. Conceptualization of the MDS Parkinson’s Disease Psychosis Rating Scale (MDS-PDPRS) prototype. [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/conceptualization-of-the-mds-parkinsons-disease-psychosis-rating-scale-mds-pdprs-prototype/. Accessed October 17, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/conceptualization-of-the-mds-parkinsons-disease-psychosis-rating-scale-mds-pdprs-prototype/