Category: Parkinson's Disease: Neuroimaging
Objective: The objective of this study is to compare robust neuromelanin (NM) substantia nigra pars compacta (SN) and locus coeruleus (LC) quantification techniques captured in NM-sensitive magnetic resonance images (MRI) in healthy controls (HC), and patients with Parkinson’s disease (PD) and REM Sleep Behaviour Disorder (RBD).
Background: Neuromelanin-sensitive MRI is established as a disease-state imaging biomarker for prodromal, early and late clinical PD [1]. Yet, the NM intensity is not standardized, with most studies manually selecting the brightest voxels [2] or some computing normalized average NM values [3]. There is need to determine the best NM score for improved analysis in PD.
Method: Cross-sectional T1 MPRAGE and NM-sensitive MRI was acquired from 59 PD (18 female, µage = 62.4, µH&Y = 1.7), 32 RBD (9 female, µage = 66.3, µH&Y = 0), and 26 healthy controls (19 female, µage = 63.2). Cognition was evaluated with the Montreal Cognitive Assessment (MoCA). Patients were evalutated with the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) (C-OPN:MP-37-2020-6170 / C-OPN; C-BIG:2017-330, 15-944-MUHC).
NM images were non-linearly aligned to the PD126 template [4] using T1s and the NIST Longitudinal Pipeline [5]. Regions of interest (ROI) were define by expert manual segmentations (SN) and conservative thresholded probabilistic atlases [6] (LC).
NM intensity was quantified by four methods: ROI intensity mean, median, 95th percentile, and maximum value. These scores were normalized by the mean background intensities of the cerebral peduncles (SN) and the pontine tegmentum (LC) and correlated with clinical data.
Results: For the SN, effect sizes between PD and HC NM scores are 1.16, 1.29, 0.94, and 0.57, respectively. For the LC, effect sizes between PD and HC NM scores are 0.44, 0.55, 0.30, and 0.31, respectively.
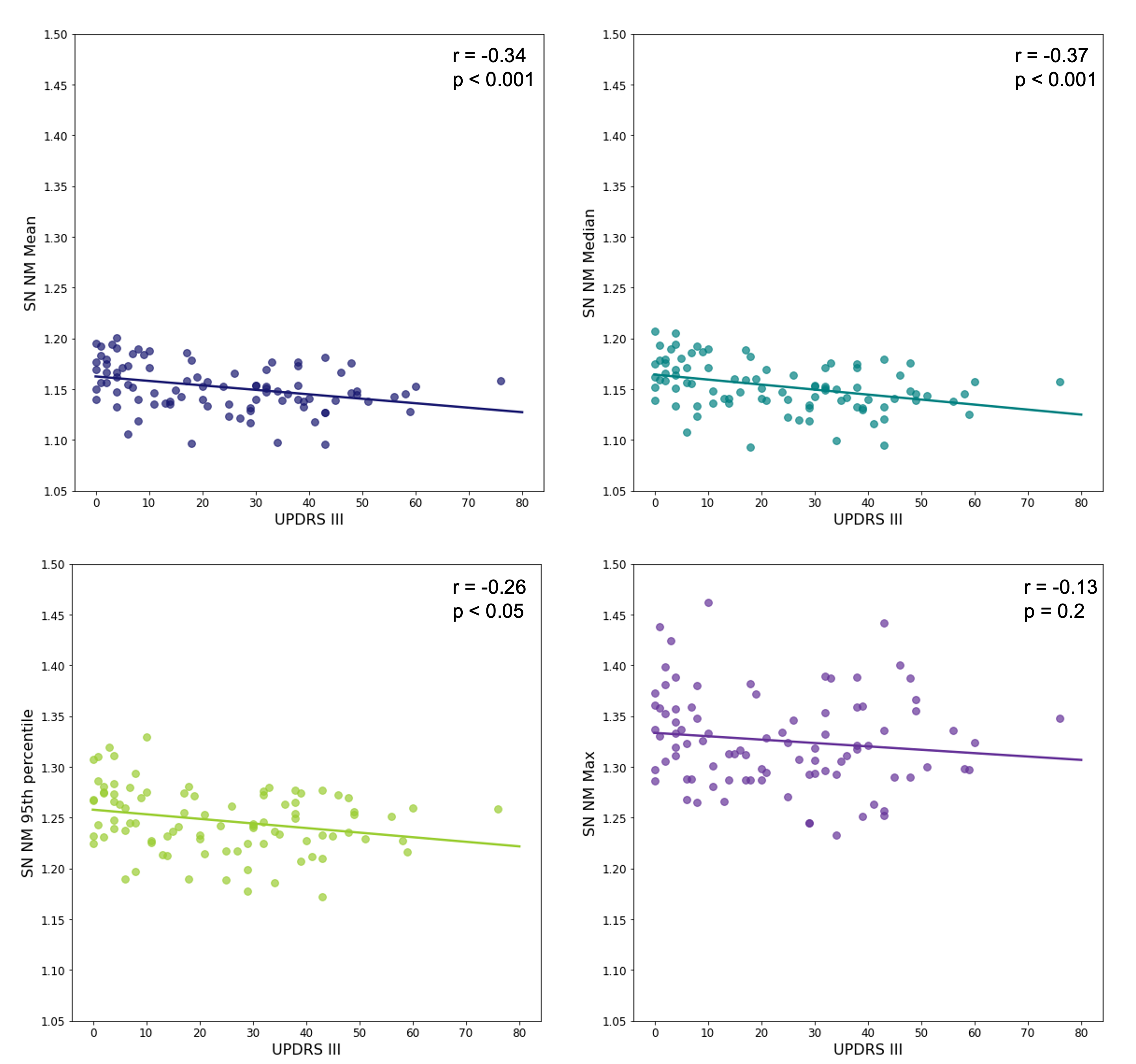
Figure 1 shows correlations between the four NM scores for the SN and MDS-UPDRS III scores. Median NM performs best (r = -0.37, p < 0.001).
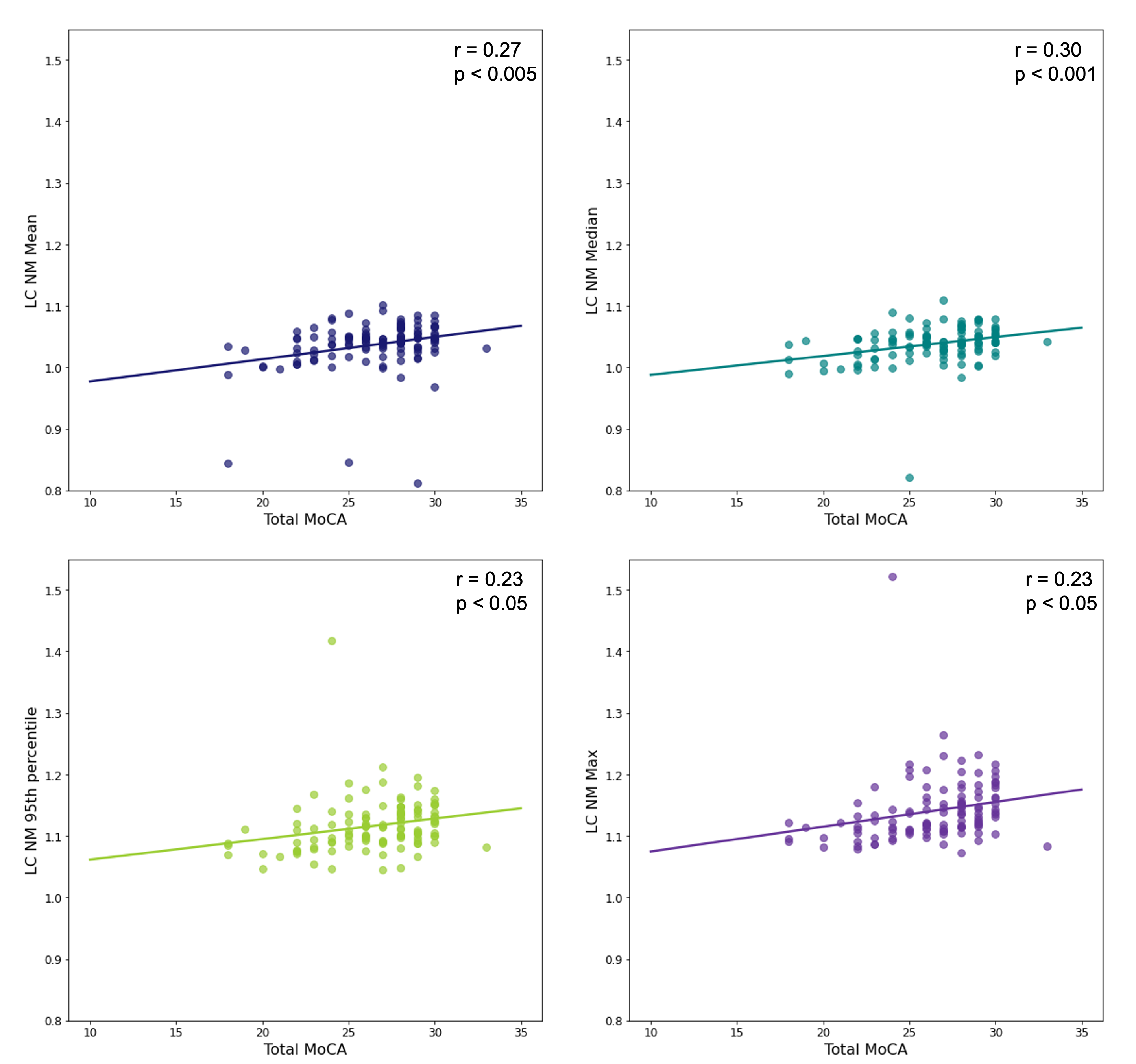
Figure 2 shows correlations between the four NM scores for the LC and total MoCA scores. Again, median NM performs best (r = 0.30, p < 0.001).
Conclusion: The automatically extracted median NM score yielded the best HC:PD effect size, and the strongest correlations between SN NM and MDS-UPDRS III, and between LC NM and cognition measured by MoCA. Correlations with MDS-UPDRS III are comparable to what is seen in the literature [7].
References: [1] T. Mitchell, S. Lehéricy, S. Y. Chiu, A. P. Strafella, A. J. Stoessl, and D. E. Vaillancourt, “Emerging Neuroimaging Biomarkers across Disease Stage in Parkinson Disease: A Review,” JAMA Neurology, vol. 78, no. 10. American Medical Association, pp. 1262–1272, 01-Oct-2021.
[2] K. Ishikuro et al., “A Parkinson’s disease patient displaying increased neuromelanin-sensitive areas in the substantia nigra after rehabilitation with tDCS: a case report,” Neurocase, vol. 27, no. 5, pp. 407–414, 2021.
[3] E. Biondetti et al., “Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease,” Brain, vol. 143, no. 9, pp. 2757–2770, Sep. 2020.
[4] V. Madge et al., “A dataset of multi-contrast unbiased average MRI templates of a Parkinson’s disease population,” medRxiv, pp. 1–8, 2022.
[5] B. Aubert-Broche et al., “A new method for structural volume analysis of longitudinal brain MRI data and its application in studying the growth trajectories of anatomical brain structures in childhood,” Neuroimage, vol. 82, pp. 393–402, 2013.
[6] M. Bianciardi et al., “Toward an In Vivo Neuroimaging Template of Human Brainstem Nuclei of the Ascending Arousal, Autonomic, and Motor Systems,” Brain Connect., vol. 5, no. 10, pp. 597–607, 2015.
[7] H. Takahashi et al., “Quantifying the severity of Parkinson disease by use of dopaminergic neuroimaging,” Am. J. Roentgenol., vol. 213, no. 1, pp. 163–168, 2019.
To cite this abstract in AMA style:
V. Madge, V. Fonov, A. Bailey, A. Dagher, E. Fon, R. Postuma, DL. Collins. Comparing neuromelanin intensity estimation techniques for Parkinson’s disease image analysis [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/comparing-neuromelanin-intensity-estimation-techniques-for-parkinsons-disease-image-analysis/. Accessed February 6, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/comparing-neuromelanin-intensity-estimation-techniques-for-parkinsons-disease-image-analysis/