Objective: To assess long-term effectiveness outcomes in people with advanced Parkinson’s Disease (aPD) on LDp/CDp versus those on oral PD therapies (standard of care, SoC).
Background: People with aPD have motor complications as their disease progresses despite optimized oral treatments. LDp/CDp is a 24-hour continuous subcutaneous infusion treatment for advanced PD and has demonstrated improvements in motor fluctuations compared to oral levodopa/carbidopa in a 12-week randomized controlled trial. Longer-term comparative outcomes to real-world SoC are lacking.
Method: Patients from a single-arm, 52-week clinical trial (NCT03781167) receiving LDp/CDp were 1:1 propensity score matched to a control group from an observational, 24-month prospective study (PROSPECT) with the same inclusion and exclusion criteria as the trial, which evaluated aPD burden on SoC. Baseline characteristics were descriptively analyzed, while ON time without troublesome dyskinesia (good ON time) and OFF time captured with PD diaries, sleep quality, quality of life (QoL), and motor experiences of daily living (m-EDL) measures were comparatively analyzed for changes from baseline to weeks 26 and 52. Comparative analyses were unadjusted and adjusted. Achievement of minimum clinically important difference (MCID) between LDp/CDp vs SoC was also assessed for all outcomes. Missing data were imputed using last observation carried forward.
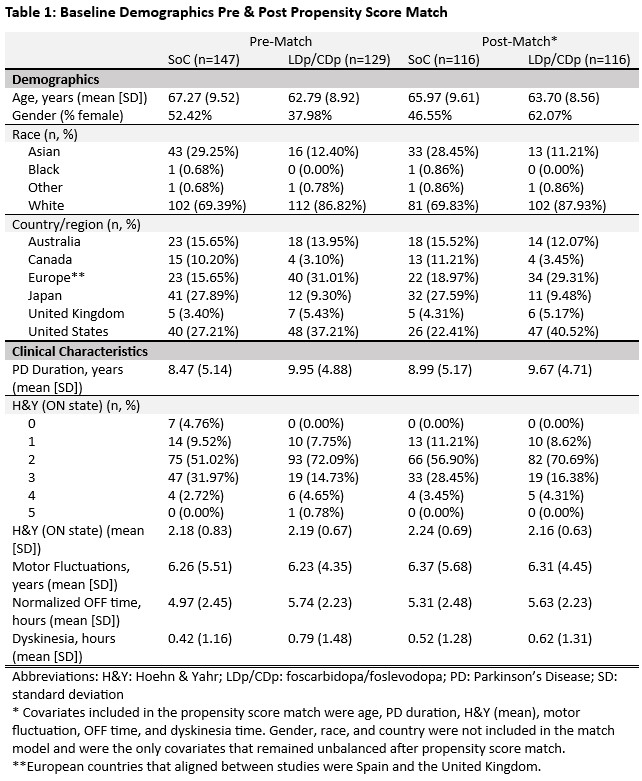
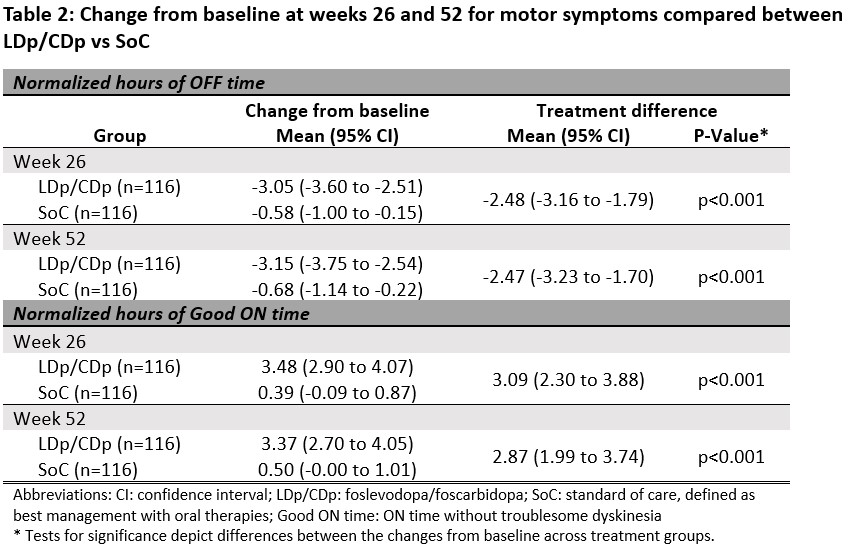
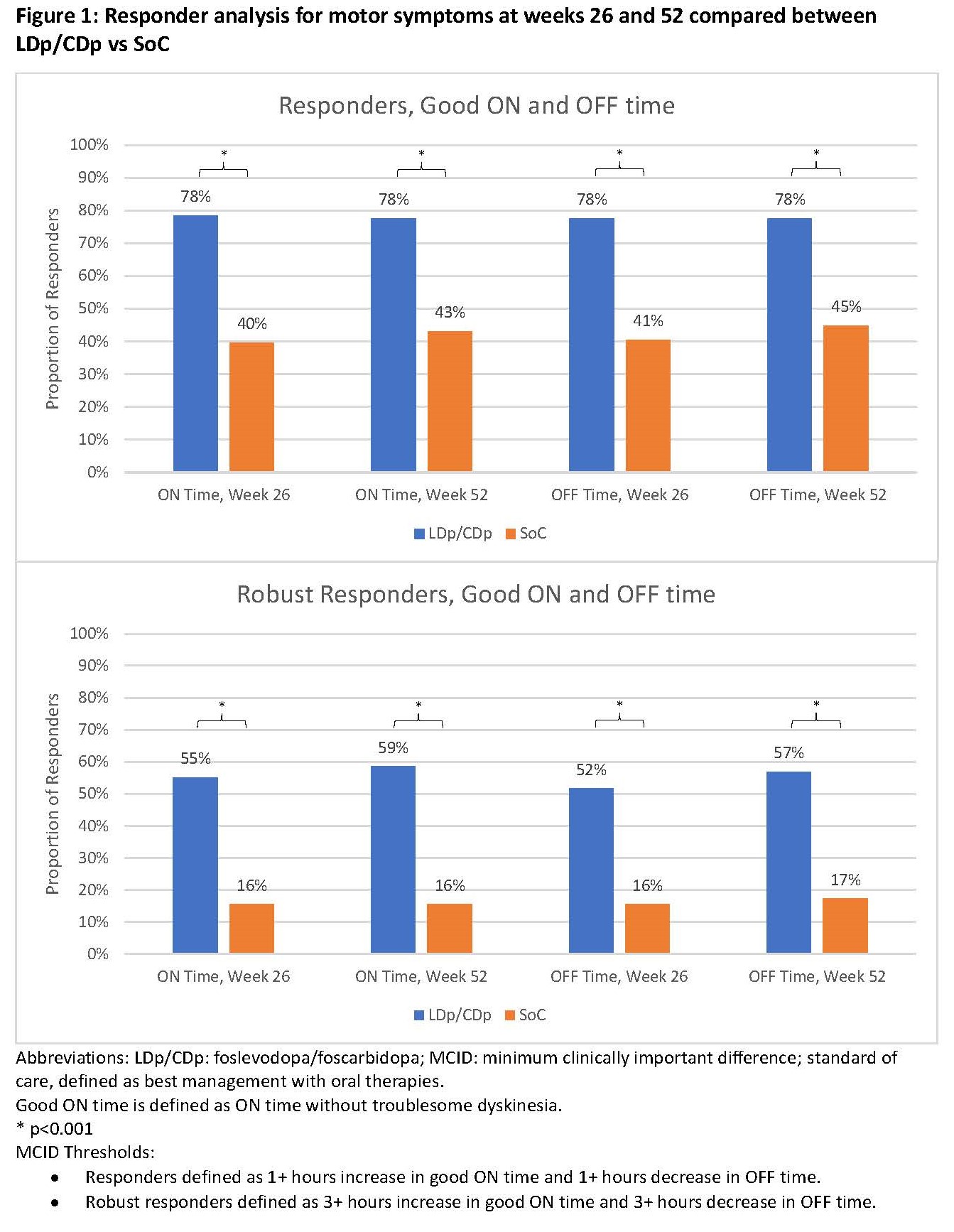
Results: 116 subjects from the LDp/CDp trial were matched to the same number of SoC subjects from PROSPECT [table 1]. LDp/CDp users had 2.47 (95% CI: -3.23, -1.70) less hours of OFF time and 2.87 (95% CI: 1.99, 3.74) more hours of good ON time at week 52 compared to SoC [table 2]. For outcomes of sleep quality, QoL, and m-EDL, there were greater improvements in change from baseline scores to weeks 26 and 52 for LDp/CDp than for SoC. The proportion of individuals reaching MCID was statistically higher for LDp/CDp compared to SoC for all outcomes at weeks 26 and 52. More than twice as many LDp/CDp patients achieved ≥3 hours of motor symptom improvement compared to SoC at weeks 26 and 52 [figure 1].
Conclusion: This indirect comparison suggests that LDp/CDp provides clinically meaningful improvements to clinical outcomes compared to real-world patients on oral therapies only for up to 52 weeks after treatment initiation.
Table 1
Table 2
Figure 1
To cite this abstract in AMA style:
D. Standaert, A. Fasano, F. Ory-Magne, D. Kern, O. de Fabregues, T. Oeda, D. Safarpour, Z. Baldwin, C. Yan, S. Wang, K. Onuk, P. Kukreja, L. Bergmann, V. Fung. Comparative-effectiveness of Foscarbidopa/foslevodopa (LDp/CDp) versus Oral Therapies in Advanced Parkinson’s Disease: A Propensity Matched Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/comparative-effectiveness-of-foscarbidopa-foslevodopa-ldp-cdp-versus-oral-therapies-in-advanced-parkinsons-disease-a-propensity-matched-study/. Accessed April 26, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/comparative-effectiveness-of-foscarbidopa-foslevodopa-ldp-cdp-versus-oral-therapies-in-advanced-parkinsons-disease-a-propensity-matched-study/