Category: Parkinson's Disease: Neuroimaging
Objective: We tested whether inter- and intra-individual differences in clinical severity are determined by cortical compensation, and not just by basal ganglia dysfunction.
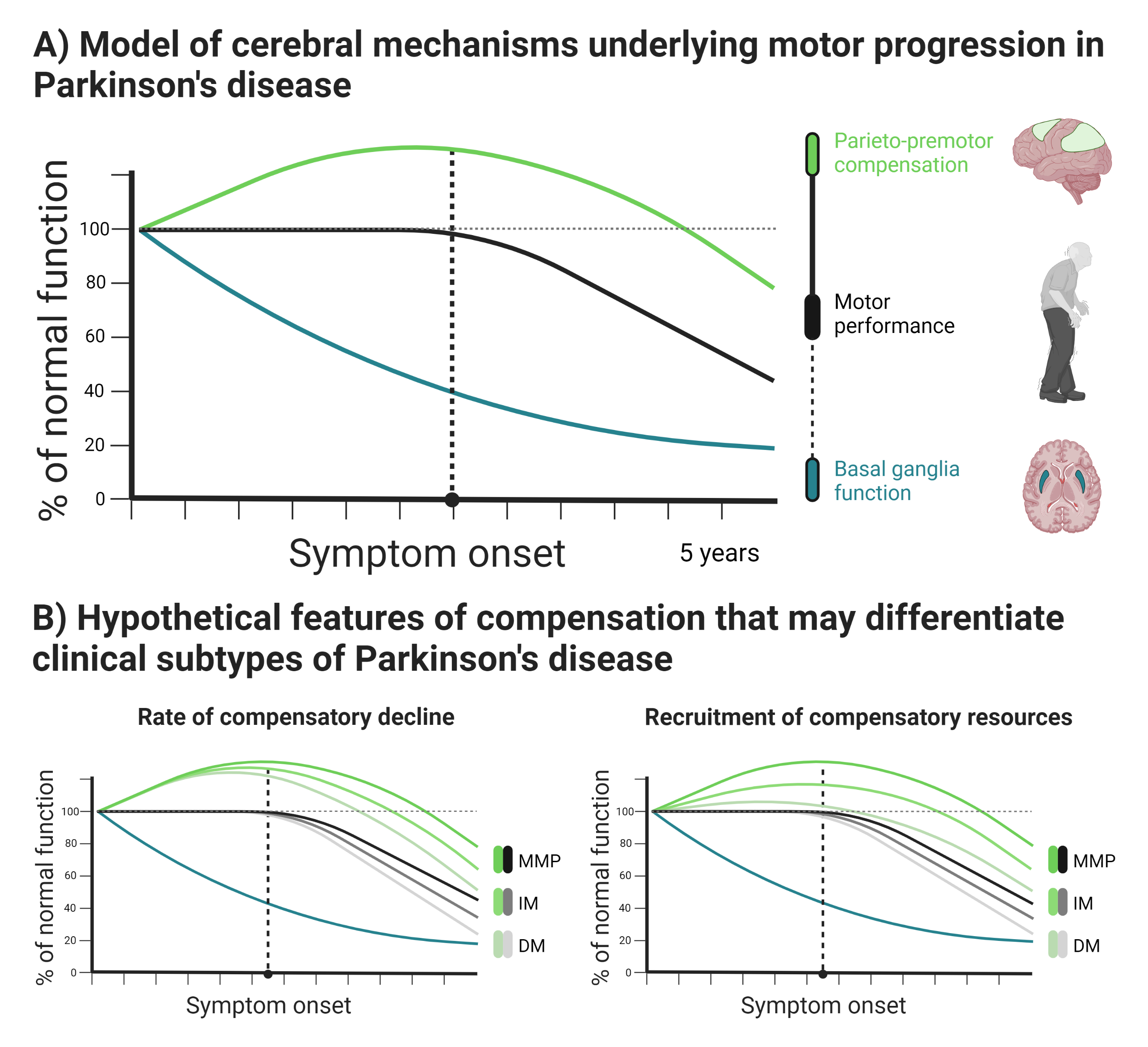
Background: Motor symptoms in Parkinson’s disease (PD) are often attributed to basal ganglia dysfunction and dopamine depletion[1]. However, dopamine depletion takes place years prior to symptom onset[2] and is a poor predictor of clinical progression[3-7]. It is therefore unlikely to be the sole determinant of motor symptoms. Functional MRI studies demonstrate that PD is associated with potentially compensatory increases in parieto-premotor activity[8-9]. However, beneficial effects of altered parieto-premotor function on motor behavior remain to be confirmed[10].
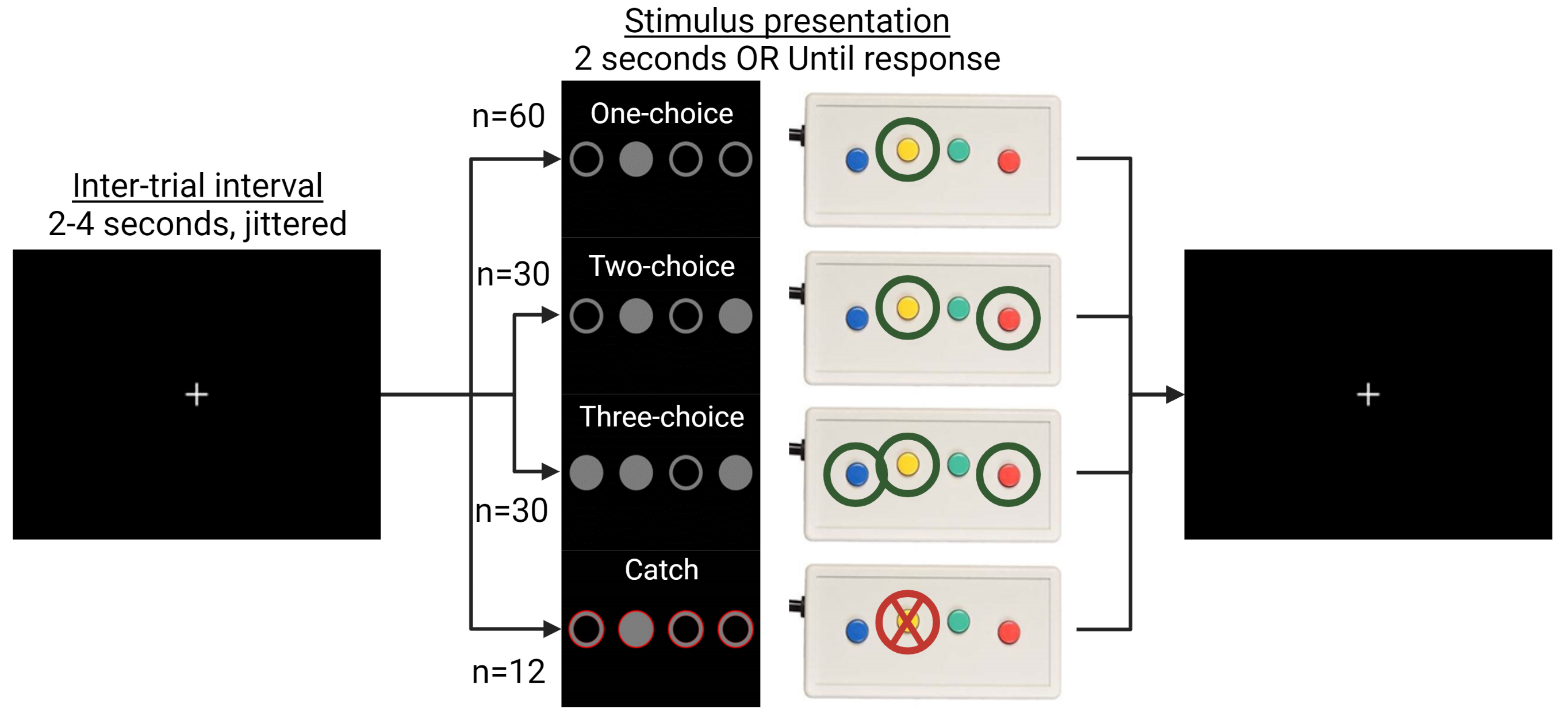
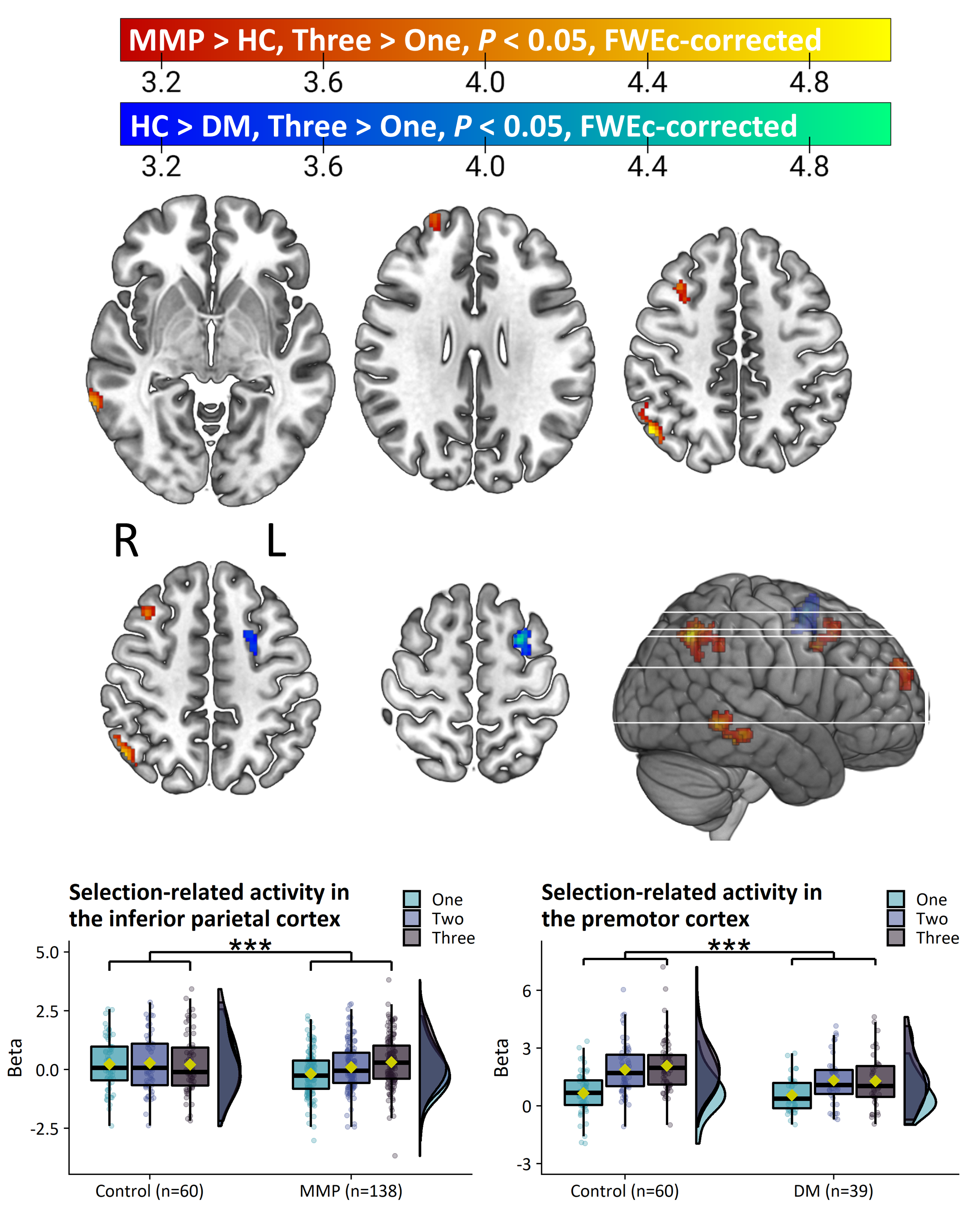
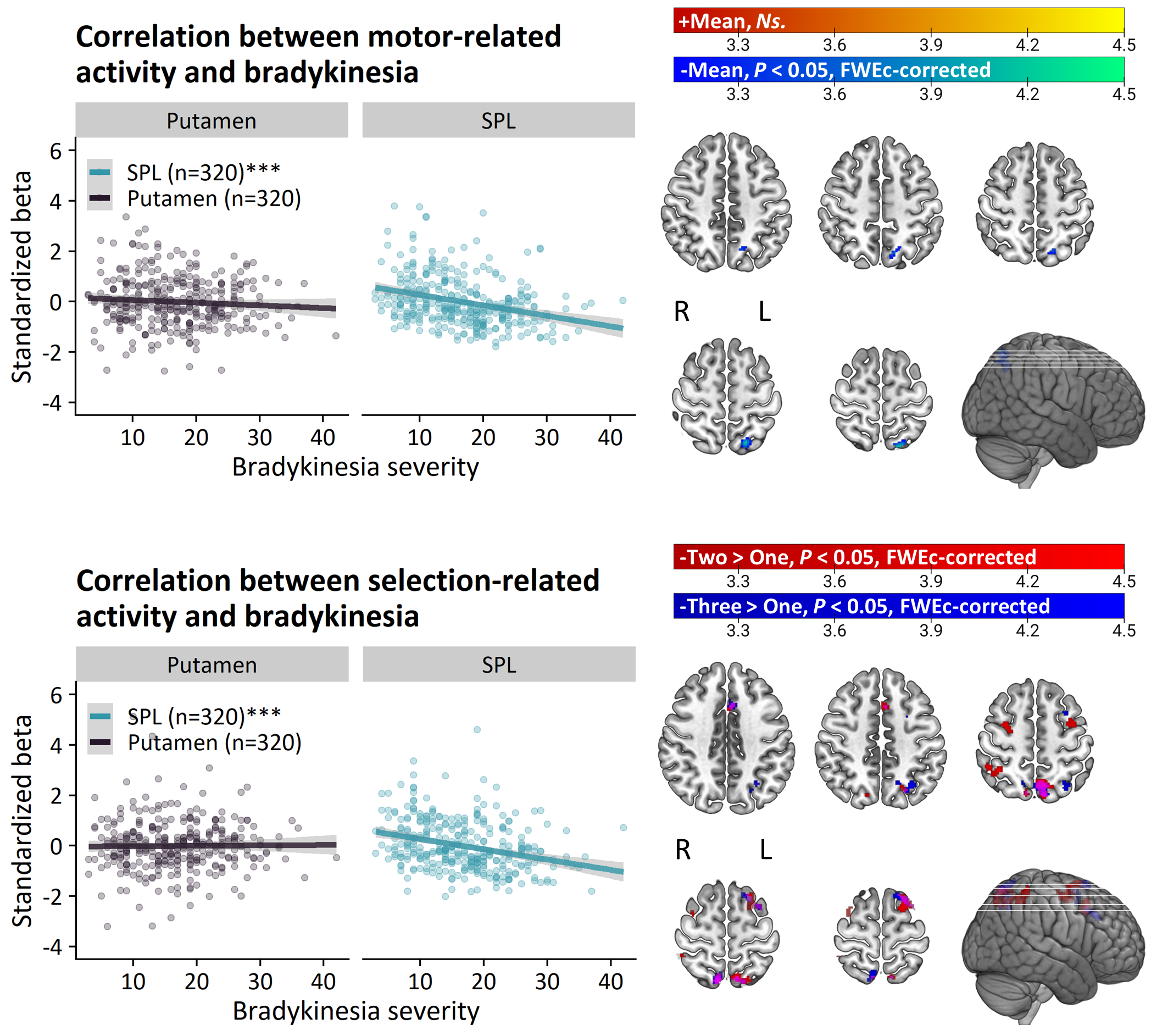
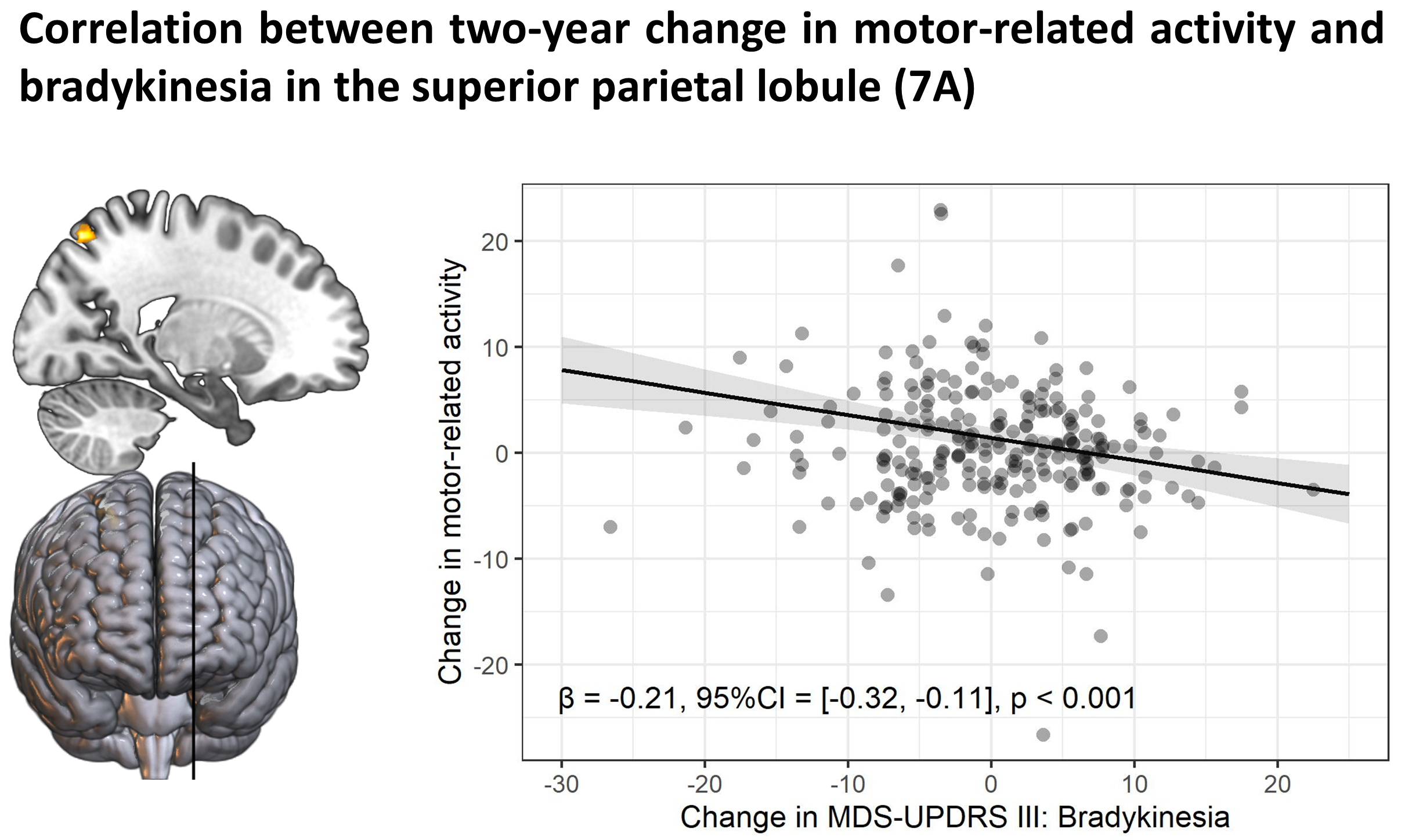
Method: 353 PD patients and 60 healthy controls from the Personalized Parkinson Project[11] performed an action selection task that is sensitive to bradykinesia during functional MRI. 326 patients and 56 healthy controls returned at two-year follow-up. The relationship between individual differences in brain activity and clinical severity was investigated in two ways. First, we compared brain activity between clinical subtypes of PD, which were defined according to a previously validated phenotyping strategy[12-14]. Second, brain activity was directly correlated with bradykinesia severity, measured with the MDS-UPDRS III. We also quantified the relationship between two-year change in bradykinesia and brain activity.
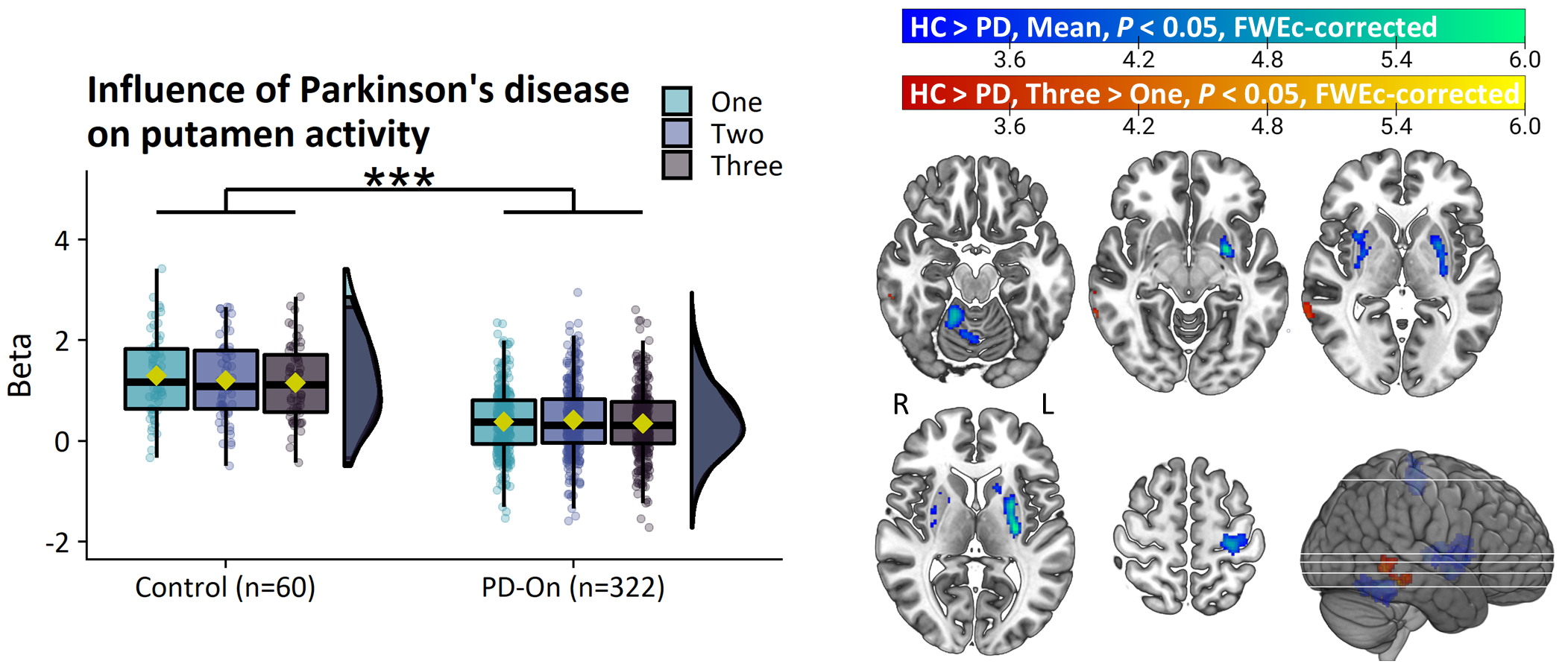
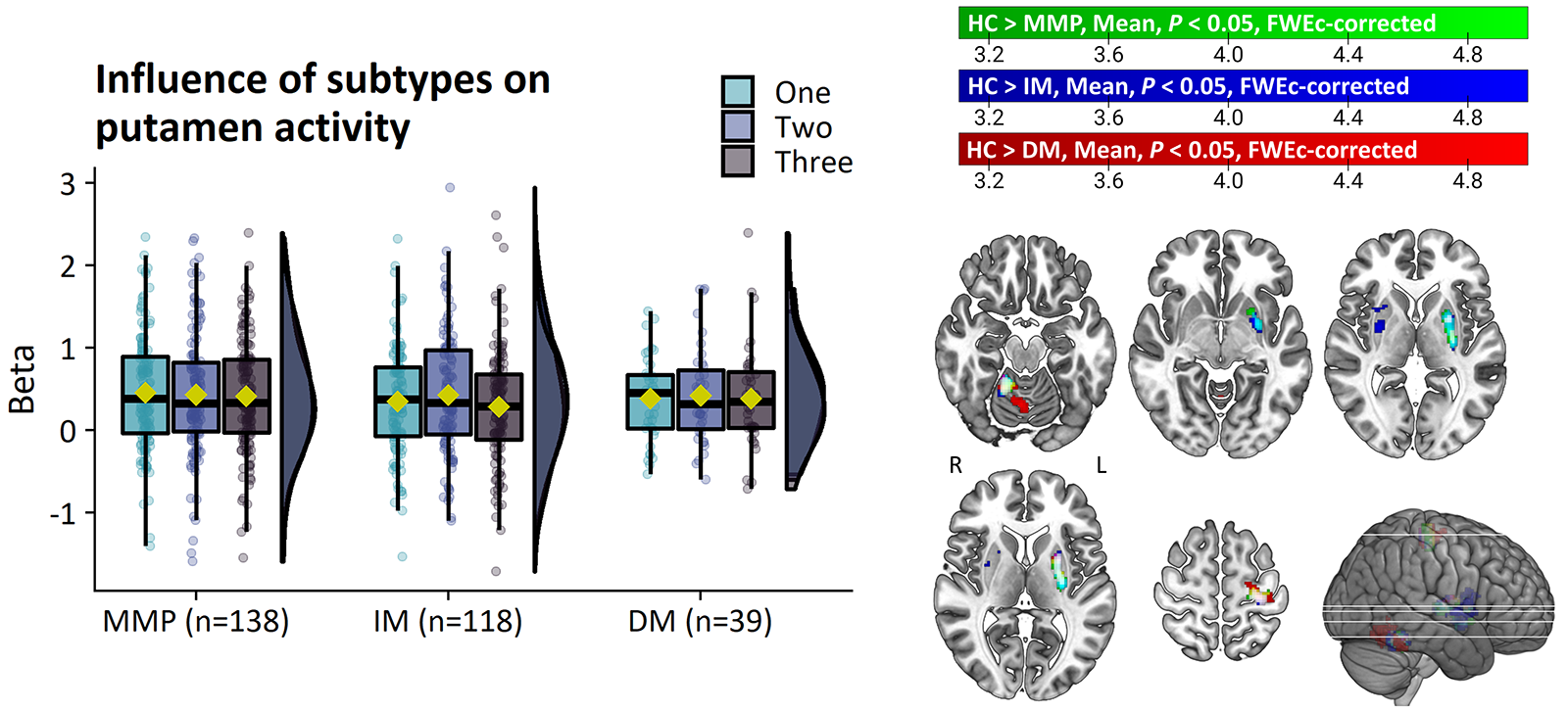
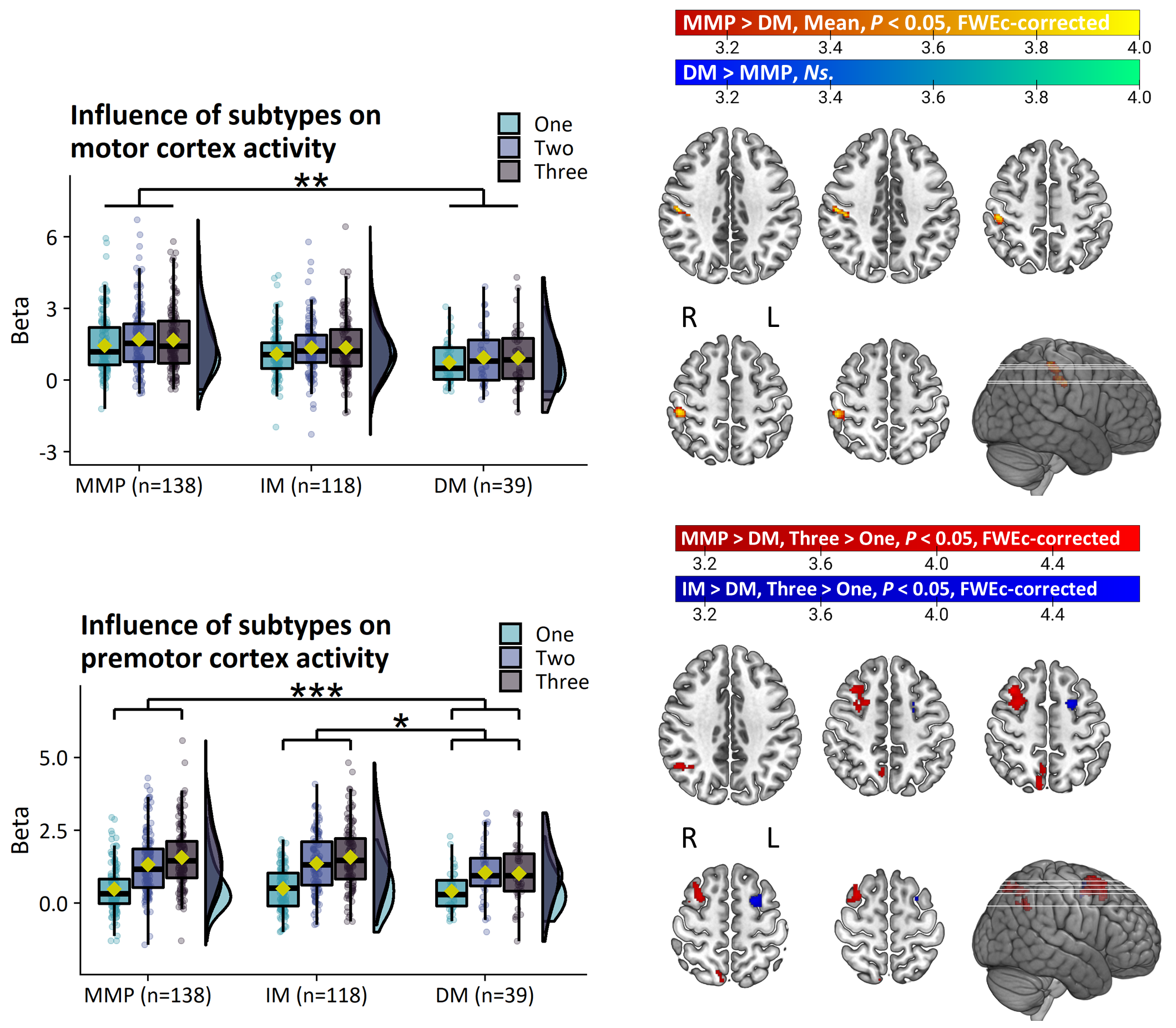
Results: PD patients had reduced basal ganglia compared to healthy controls. However, basal ganglia activity did not differ between subtypes and did not predict bradykinesia severity. In contrast, the relatively benign mild-motor predominant subtype was characterized by increased parieto-premotor activity compared to both a more severely affected diffuse-malignant subtype and healthy controls. Consistently, parieto-premotor activity inversely correlated with bradykinesia severity. Additionally, two-year change in superior parietal cortex activity inversely correlated with bradykinesia progression.
Conclusion: In conclusion, our findings strongly support a compensatory role of the parieto-premotor cortex and indicates that decline in cortical compensation may be a critical determinant of clinical severity and progression in PD. Treatments that maintain or enhance cortical compensation may serve as a valuable complement to traditional dopaminergic medication.
References: 1. Obeso JA, Stamelou M, Goetz CG, et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord. 2017;32(9):1264-1310. doi:10.1002/mds.27115
2. Fearnley JM, Lees AJ. Ageing and Parkinson’s Disease: Substantia Nigra Regional Selectivity. Brain. 1991;114(5):2283-2301. doi:10.1093/brain/114.5.2283
3. Nurmi E, Bergman J, Eskola O, et al. Progression of dopaminergic hypofunction in striatal subregions in Parkinson’s disease using [18F]CFT PET. Synapse. 2003;48(3):109-115. doi:10.1002/syn.10192
4. Nurmi E, Ruottinen HM, Bergman J, et al. Rate of progression in Parkinson’s disease: A 6-[18F]fluoro-L-dopa PET study. Mov Disord. 2001;16(4):608-615. doi:10.1002/mds.1139
5. Pirker W, Holler I, Gerschlager W, Asenbaum S, Zettinig G, Brücke T. Measuring the rate of progression of Parkinson’s disease over a 5-year period with β-CIT SPECT. Mov Disord. 2003;18(11):1266-1272. doi:10.1002/mds.10531
6. Morrish PK, Rakshi JS, Bailey DL, Sawle G V, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry. 1998;64(3):314-319. doi:10.1136/jnnp.64.3.314
7. Morrish PK, Sawle G V., Brooks DJ. An [ 18 F]dopa–PET and clinical study of the rate of progression in Parkinson’s disease. Brain. 1996;119(2):585-591. doi:10.1093/brain/119.2.585
8. Herz DM, Eickhoff SB, Løkkegaard A, Siebner HR. Functional neuroimaging of motor control in parkinson’s disease: A meta-analysis. Hum Brain Mapp. 2014;35(7):3227-3237. doi:10.1002/hbm.22397
9. Herz DM, Meder D, Camilleri JA, Eickhoff SB, Siebner HR. Brain Motor Network Changes in Parkinson’s Disease: Evidence from Meta-Analytic Modeling. Mov Disord. 2021;36(5):1180-1190. doi:10.1002/mds.28468
10. Cabeza R, Albert M, Belleville S, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19(11):701-710. doi:10.1038/s41583-018-0068-2
11. Bloem BR, Marks WJ, Silva de Lima AL, et al. The Personalized Parkinson Project: examining disease progression through broad biomarkers in early Parkinson’s disease. BMC Neurol. 2019;19(1):1-10. doi:10.1186/s12883-019-1394-3
12. Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson’s disease: Biomarkers and longitudinal progression. Brain. 2017;140(7):1959-1976. doi:10.1093/brain/awx118
13. De Pablo-Fernández E, Lees AJ, Holton JL, Warner TT. Prognosis and Neuropathologic Correlation of Clinical Subtypes of Parkinson Disease. JAMA Neurol. 2019;76(4):470-479. doi:10.1001/jamaneurol.2018.4377
14. Johansson ME, van Lier NM, Kessels RPC, Bloem BR, Helmich RC. Two-year clinical progression in focal and diffuse subtypes of Parkinson’s disease. npj Park Dis. 2023;9(1):29. doi:10.1038/s41531-023-00466-4
To cite this abstract in AMA style:
M. Johansson, I. Toni, R. Kessels, B. Bloem, R. Helmich. Clinical severity in Parkinson’s disease is determined by decline in cortical compensation [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/clinical-severity-in-parkinsons-disease-is-determined-by-decline-in-cortical-compensation/. Accessed December 27, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/clinical-severity-in-parkinsons-disease-is-determined-by-decline-in-cortical-compensation/