Objective: The objective is to investigate the clinical outcomes and subsequent use of medications in a group of Colombian Patients with DBS Surgery.
Background: Parkinson’s disease has increased its prevalence in Colombia as in the rest of the world, due to several factors, including population aging.
This has led to the strengthening for therapies such as DBS, which are known to be effective in symptom control and subsequently in improvement quality of life. However, in Colombia little is known about the impact of said therapy in terms of symptomatic control. For that reason, this study aims to investigate the outcomes of Parkinson’s disease DBS patients in a multicentric cohort in Colombia.
Method: A Cohort study was conducted with 394 patients including 253 males and 141 females with Parkinson’s disease Diagnostic who underwent bilateral DBS (STN and GPi) at multiple institutions in Colombia. Preoperative and postoperative follow-up assessments and comparisons between UPDRS III and medications doses were analyzed. We used Wilcoxon test to compare pre and post operative UPDRS III and reduction in medication use, providing statistical analysis of the efficacy of DBS.
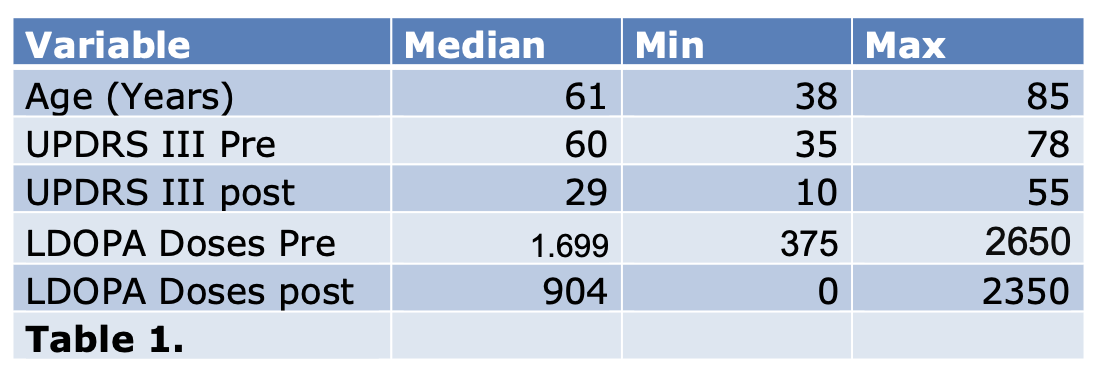
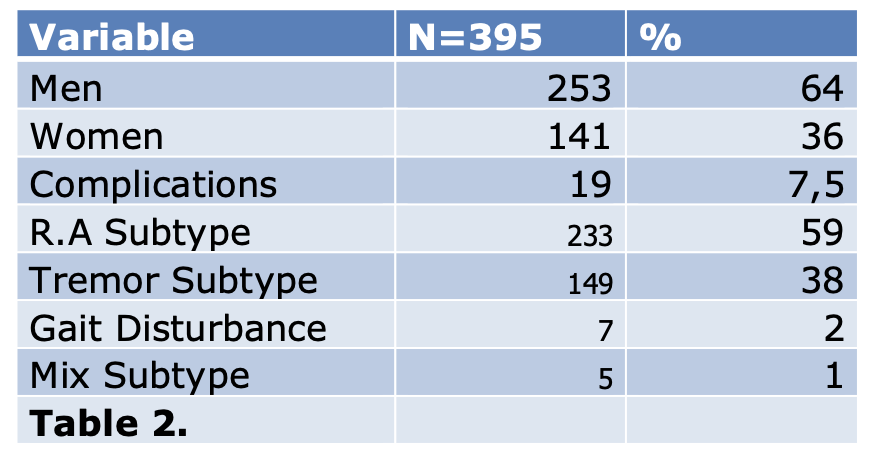
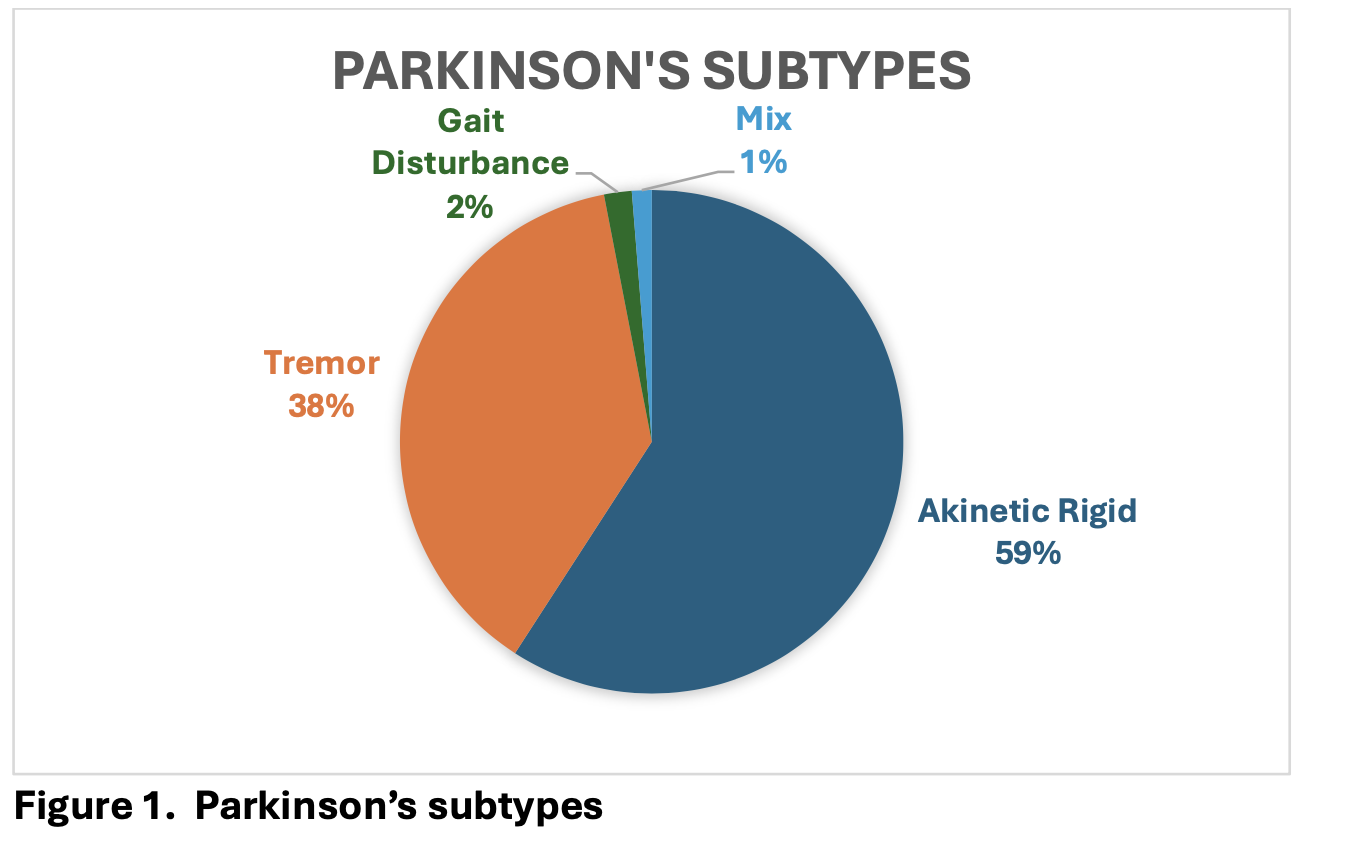
Results: The mean age at the time of surgery was 61 (range 38-85) years. 149 patients have tremor dominant subtype ( 37,8%), 233 have akinetic rigid dominant subtype ( 59,15%) , 7 have gait disturbance subtype ( 1,7%) and 5 patients have mixed subtype (1,26%). (Table 1) (Figure 1)
19 patients had complications after surgery (7,5%), 2 patients (0,5%) died during the follow up period. 16 patients (4%) suffer impulsivity after the surgery. 1 patient (0,25%) have Dementia diagnosis after surgery. (Table 2)
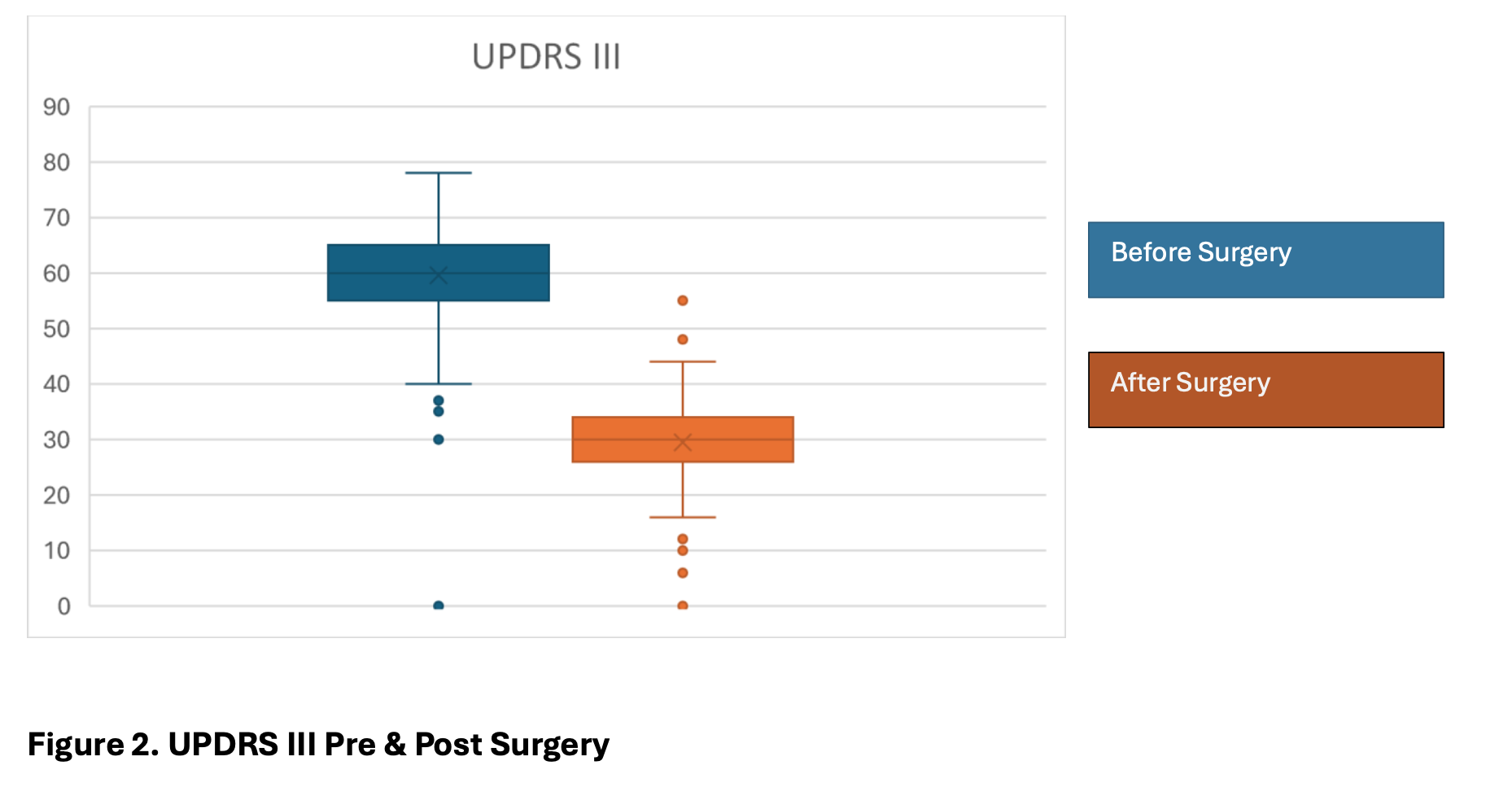
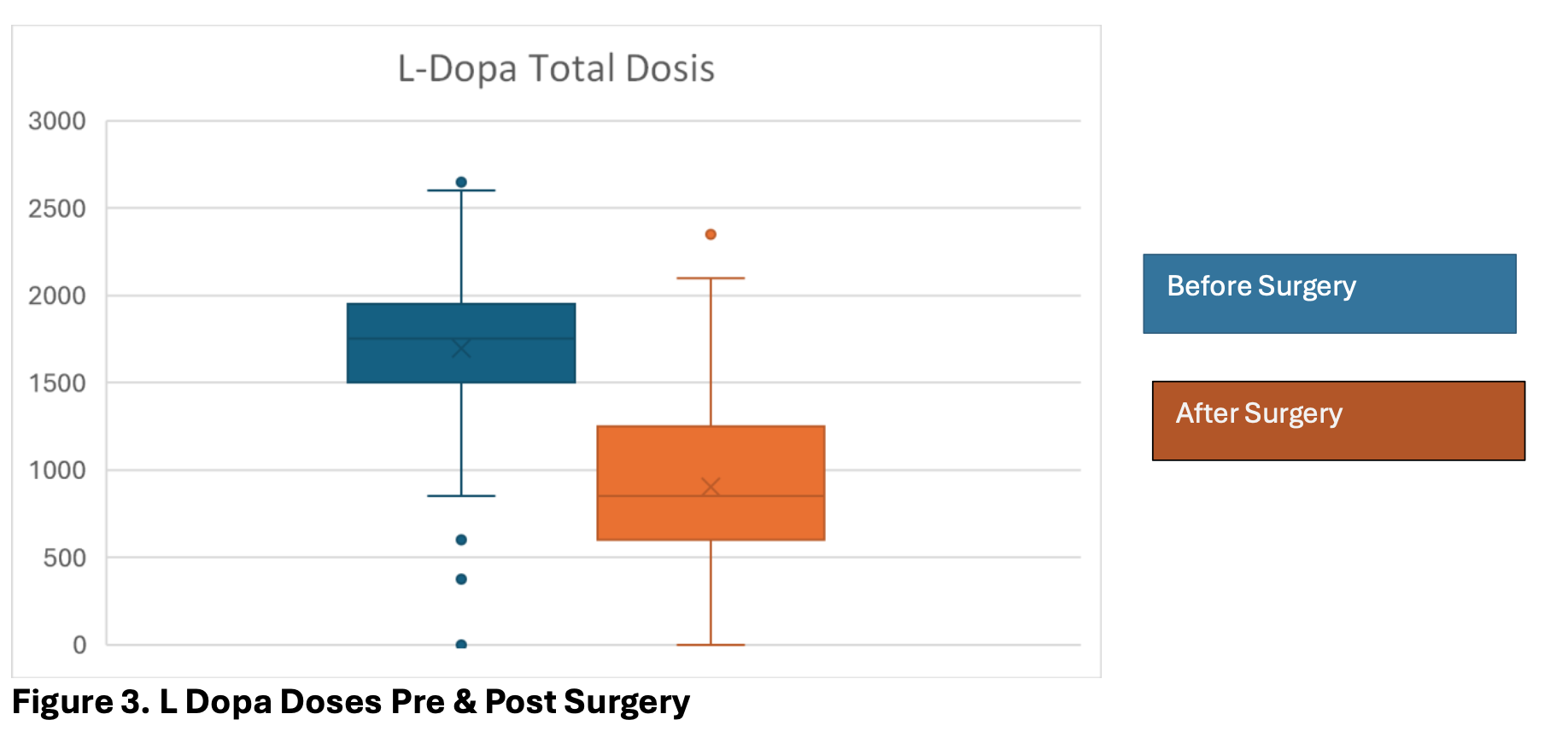
The UPDRS score was significantly improved after surgery with a mean of 29,54 (range 10 – 55). UPDRS III pre surgery had a mean of 60 (range 35 – 78), Which shows an improvement of 51% in the scale. (Figure 2, 3)
The mean reduction of medications was 47% in the total dose of L-Dopa (range 0-100) after surgery, managing to suspend medication for 20 patients (5%) ( Table 2)
Conclusion: DBS is a safe and effective treatment for Colombian’s patients. It significantly reduces the UPDRS III score, reduces medication intake and in our cohort does not present a high rate of adverse events, with a very low mortality, as well as a low rate of impulsivity and subsequent cognitive decline.
Table 1.
Table 2.
Figure 1. Parkinson’s Subtypes
Figure 2. UPDRSIII Pre and post Surgery
Figure 3. L Dopa Doses pre and post Surgery
References: 1. The Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956–63.
2. Engelhardt J, Caire F, Damon-Perrière N, Guehl D, Branchard O, Auzou N, et al. A phase 2 randomized trial of asleep versus awake subthalamic nucleus deep brain stimulation for Parkinson’s disease. Stereotact Funct Neurosurg. 2021;99:230–40.
3. Dafsari HS, Dos Santos Ghilardi MG, Visser-Vandewalle V, Rizos A, Ashkan K, Silverdale M, et al. Beneficial nonmotor effects of subthalamic and pallidal neurostimulation in Parkinson’s disease. Brain Stimul. 2020;13:1697–705.
4. Limousin P, Foltynie T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat Rev Neurol. 2019;15:234–42.
To cite this abstract in AMA style:
T. Lopez Gonzalez, S. Poveda, M. Fonseca, P. Arango, O. Rojas, G. Monsalve, JC. Diez, J. Lobato, O. Bernal Pacheco. Clinical Response and Reduction in Medication Use After Deep Brain Stimulation Surgery in a Cohort of Parkinson’s Disease Patients in Colombia [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/clinical-response-and-reduction-in-medication-use-after-deep-brain-stimulation-surgery-in-a-cohort-of-parkinsons-disease-patients-in-colombia/. Accessed February 6, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/clinical-response-and-reduction-in-medication-use-after-deep-brain-stimulation-surgery-in-a-cohort-of-parkinsons-disease-patients-in-colombia/