Category: Parkinson's Disease: Genetics
Objective: To design a model for prediction of GBA carrier status based on clinical and demographic features in a cohort of PD patients.
Background: Given the unique natural history of GBA-related Parkinson’s disease (GBA-PD) and the potential for novel treatments in the GBA-PD population, identifying these patients early is crucial for prognostication and stratification for clinical trials [1-2]. Clinically distinguishing GBA-PD from idiopathic PD on an individual level is challenging. A simplified model for predicting GBA carrier status would prove useful to help target genetic testing in clinical settings [3].
Method: We performed an in-depth clinical characterization of a cohort of subjects with PD (n = 100) using standardized rating scales for motor and non-motor symptoms of PD and self-reported information for the same traits. The results of the two data collections were compared. We then utilized these clinical features to generate a predictive model for detecting the presence of GBA mutations, first in a cohort of PD patients from our center (study cohort model), and then in a larger cohort of subjects with PD from the Parkinson’s Progression Marker Initiative (n = 420) (PPMI cohort model).
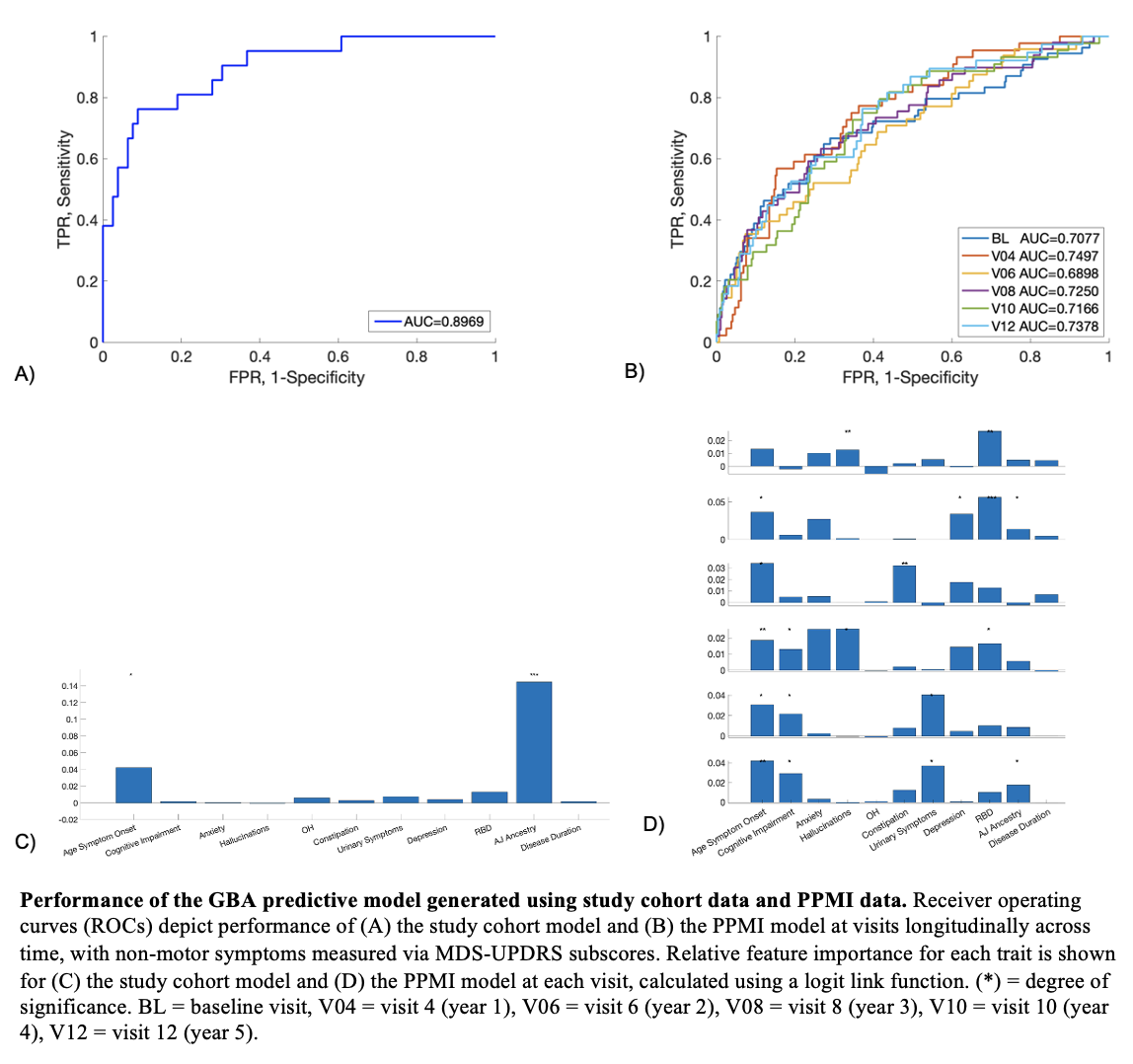
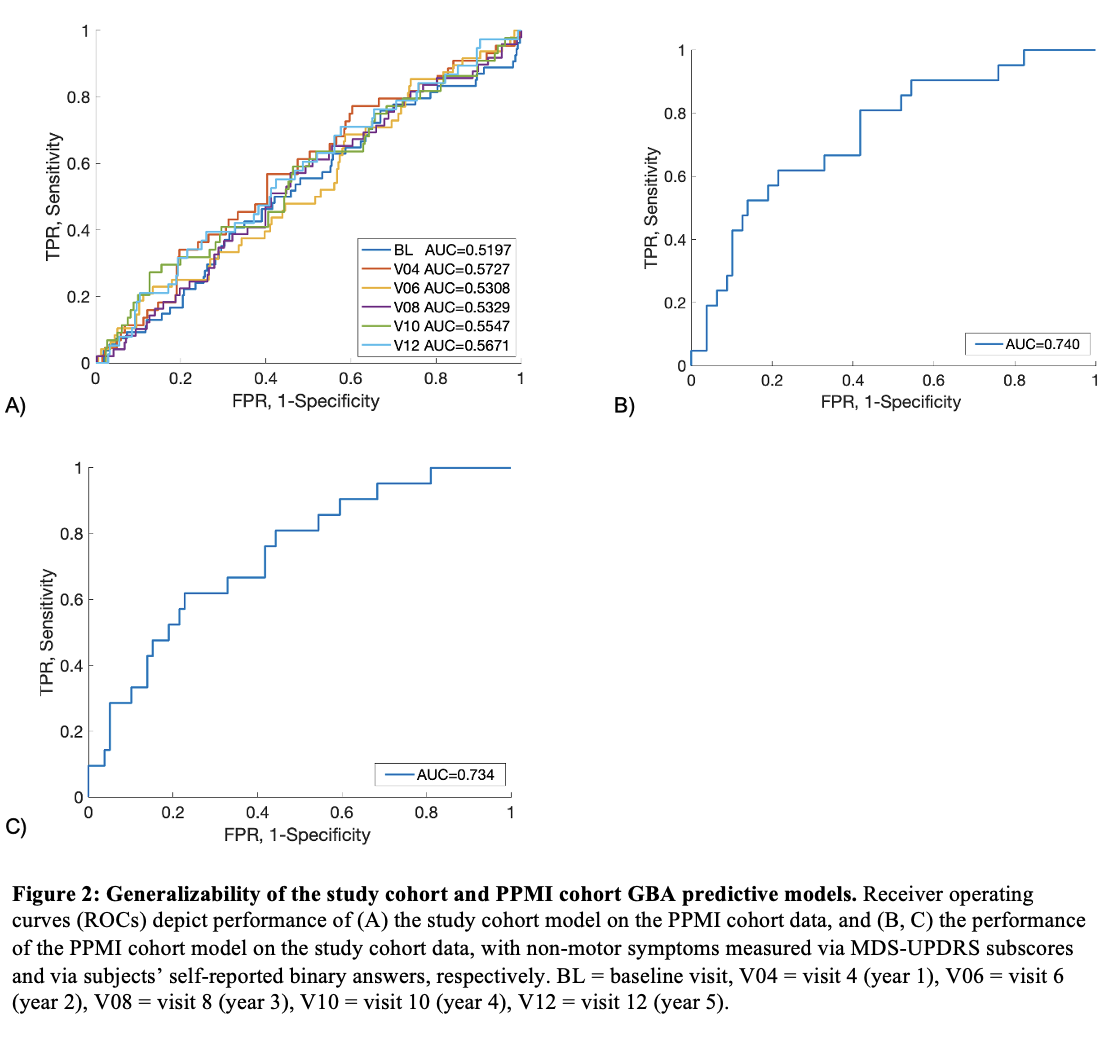
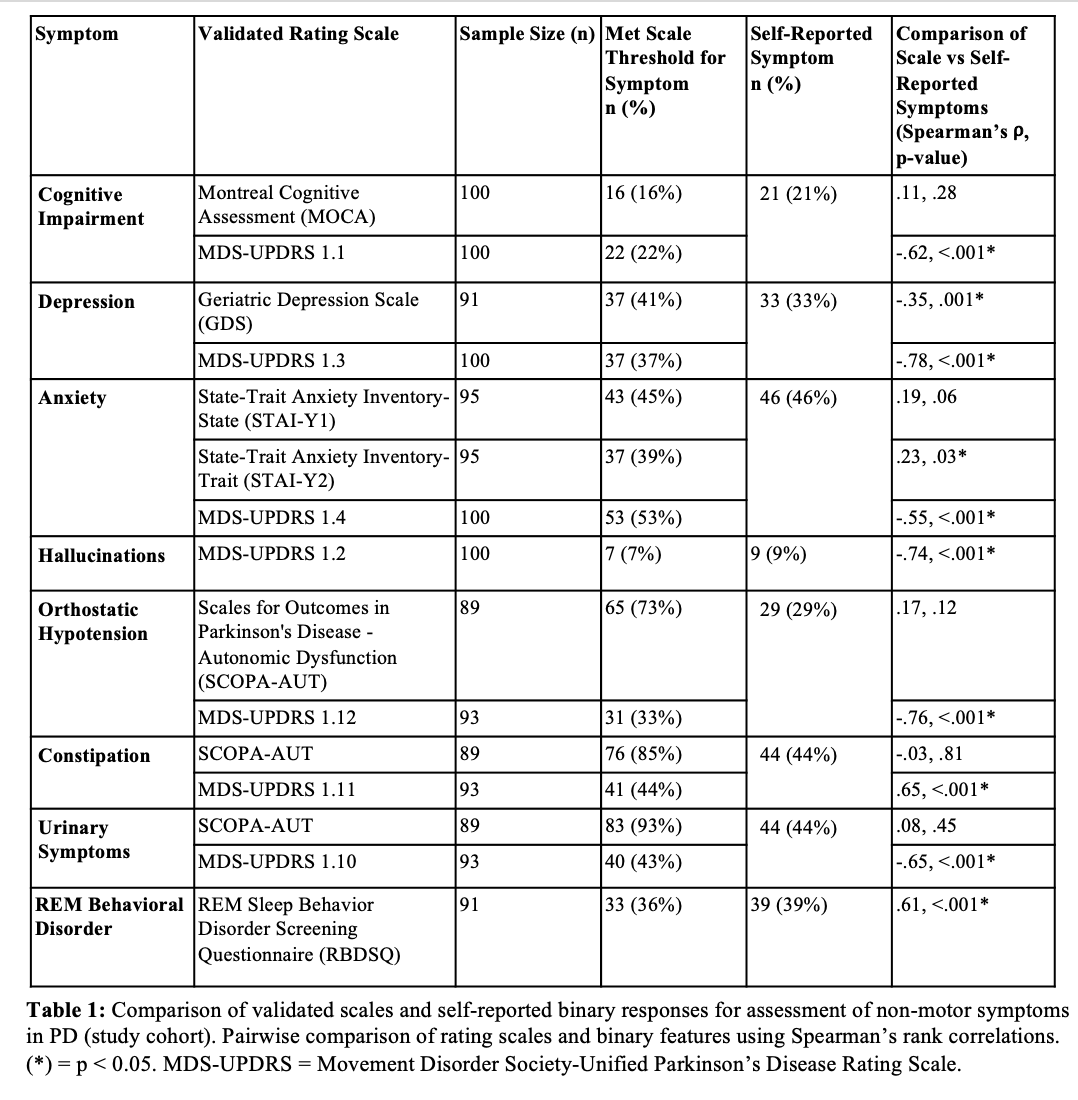
Results: The study cohort and PPMI cohort predictive models successfully discriminated GBA variant status in their respective cohorts, with predictive values of 0.897 and 0.738, respectively (Figure 1A, 1B) [Figure 1]. Ashkenazi Jewish (AJ) ancestry represented the only statistically significant driver of the study cohort model, whereas AJ ancestry, age of symptom onset, cognitive impairment, and urinary symptoms contributed significantly to the PPMI cohort model’s performance (Figure 1C, 1D). The PPMI cohort model successfully generalized but the study cohort model did not, possibly due to small sample size (Figure 2A, 2B). We also showed that self-reported binary responses reliably correlate with outcomes on standardized rating scales for most non-motor symptoms in PD, and the PPMI model performs equally well on this binomial data (AUC 0.734) (Figure 2C) [Table 1].
Conclusion: We describe a simple, clinically oriented statistical model for prediction of GBA carrier status in the general PD population. In the future, this model can be implemented in order to help target genetic testing by selecting patients with clinical features that are highly associated with GBA variants.
References: 1. Blandini F, et al. Glucocerebrosidase mutations and synucleinopathies: Toward a model of precision medicine. Mov Disord. 2019; 34(1): p. 9-21.
2. Menozzi, E. and A.H.V. Schapira, Exploring the Genotype-Phenotype Correlation in GBA-Parkinson Disease: Clinical Aspects, Biomarkers, and Potential Modifiers. Front Neurol, 2021. 12: p. 694764.
3. Winder-Rhodes, S.E., et al., Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain, 2013. 136(Pt 2): p. 392-
To cite this abstract in AMA style:
J. Greenberg, K. Astudillo, S. Frucht, A. Flinker, G. Riboldi. Clinical Prediction of GBA Carrier Status in Parkinson’s Disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/clinical-prediction-of-gba-carrier-status-in-parkinsons-disease/. Accessed January 7, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/clinical-prediction-of-gba-carrier-status-in-parkinsons-disease/