Category: Parkinson's Disease: Genetics
Objective: Describe a detailed clinical and genetic evaluation of patients with Parkinson’s disease secondary to DJ1 gene mutation
Background: Inherited Parkinson’s disease (PD) represents 5-10% of PD patients. The International Parkinson’s Disease and Movement Disorder Society Gene Database currently contains more than 1100 gene variants in movement disorder patients (MDSGene). (1) The MDSGene contains 33 patients with DJ1 gene mutation causing AR early-onset PD. (2) There has been reported concern regarding the lack of detailed description and phenotypic data of DJ1 mutation associated with young onset PD. (3)
Method: We included all patients diagnosed with PD and confirmed genetic mutation in the DJ1 gene of any age. We retrospectively obtained the data using the patient’s chart reviews and videos from the Movement Disorder Video Library. We organized, cleaned, and analyzed the dataset using SPSS.
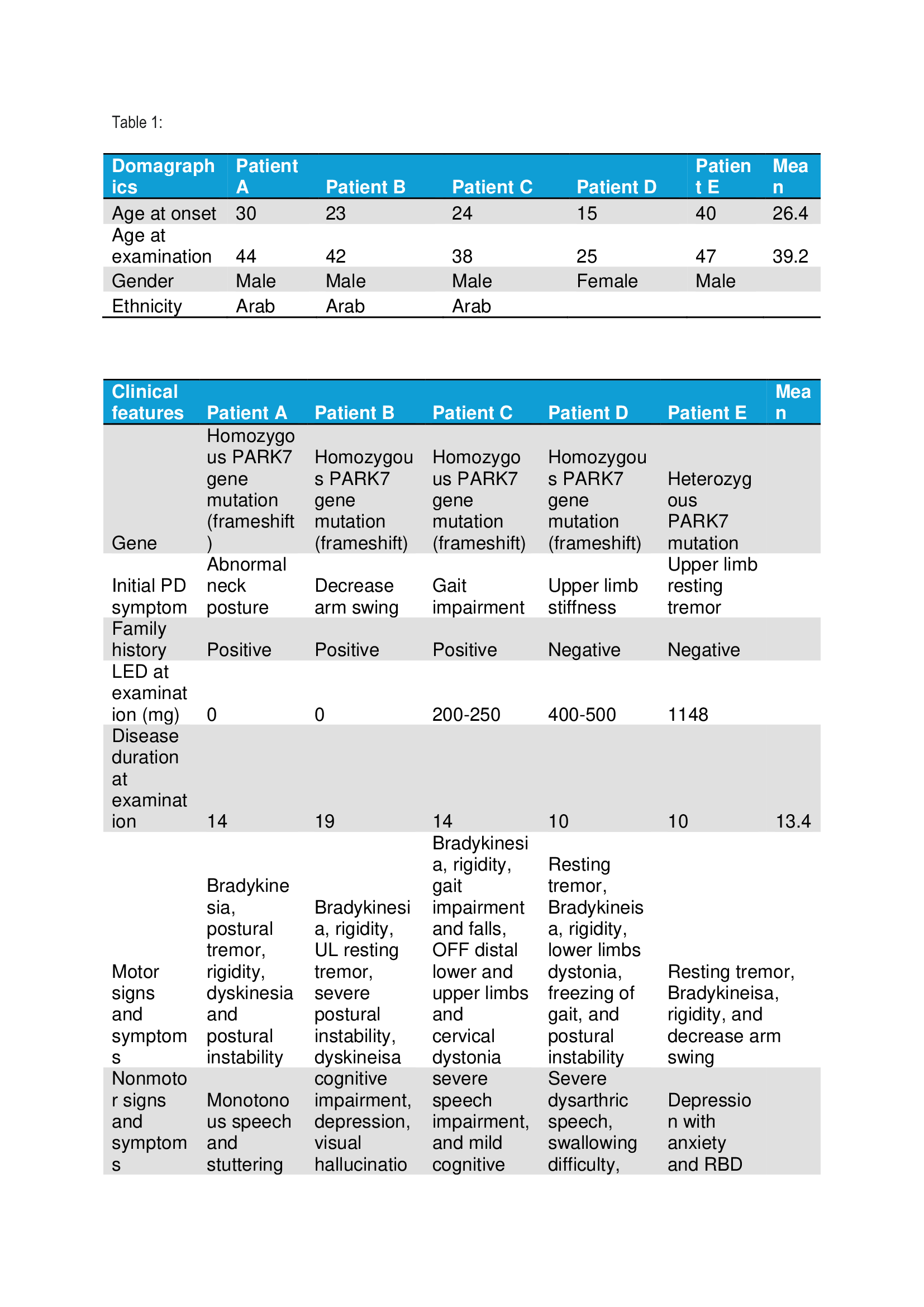
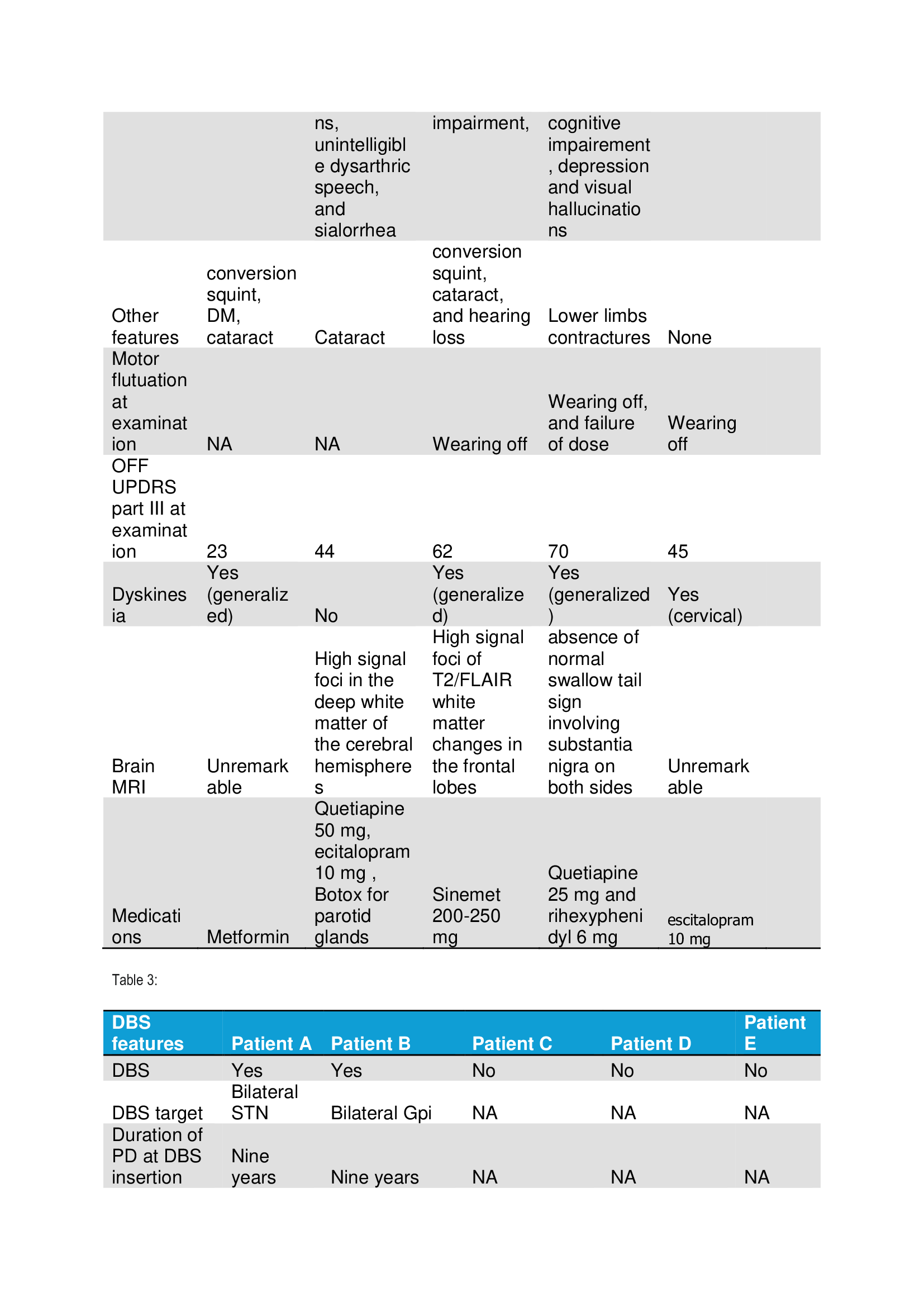
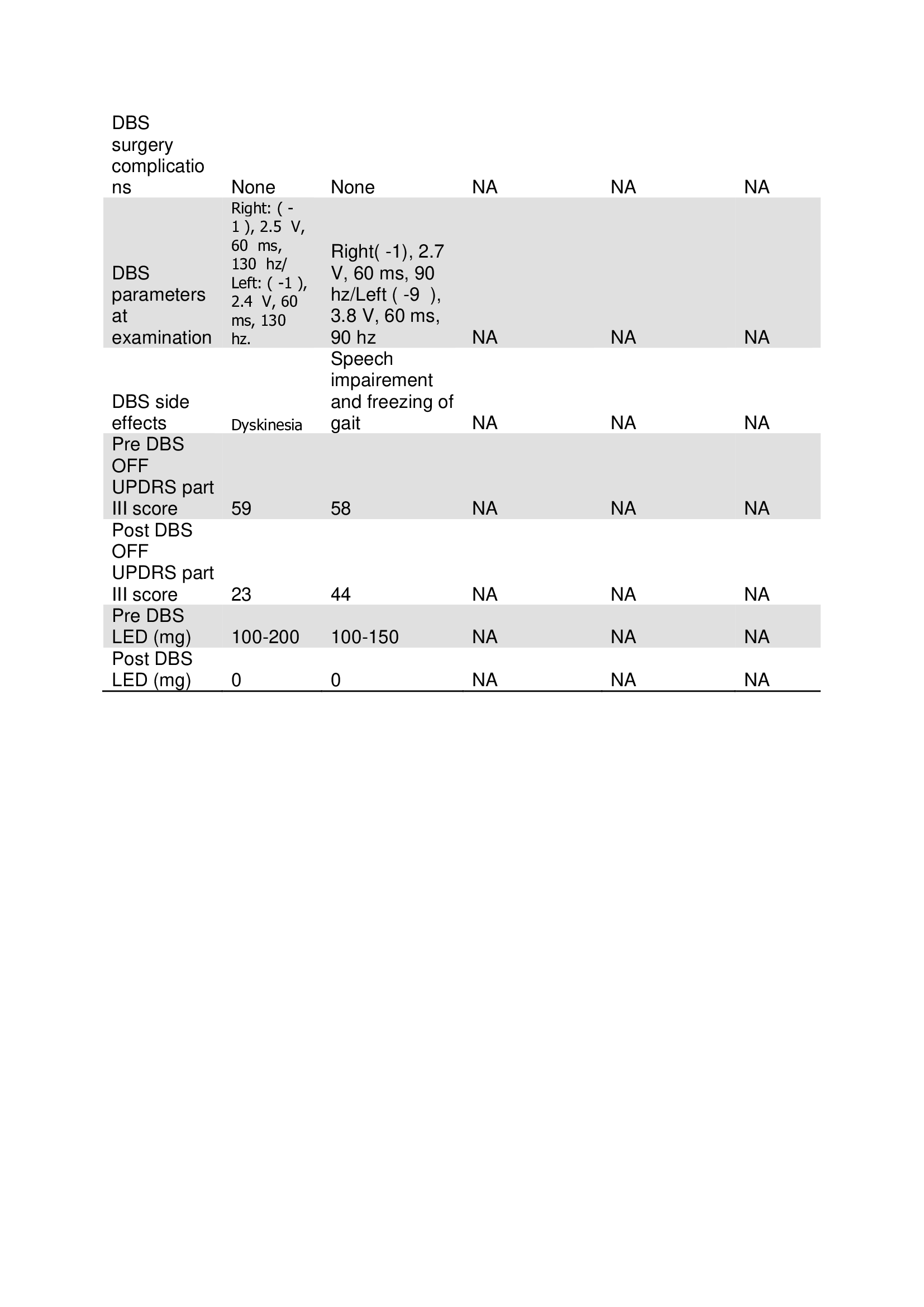
Results: We included five patients with PD secondary to PARK7 mutation. Four patients had a frameshift homozygotic mutation of the PARK7 gene. The mean age in years at disease onset was 26.4, and the disease duration was 13.4. Four patients were males, and three patients had a positive family history. (Table 1) All patients had good responses to levodopa, and four patients had severe levodopa-induced dyskinesia. Motor fluctuations and dependency developed nine to ten years after the onset of the symptoms. The motor symptoms included bradykinesia, rigidity, resting or postural tremor, freezing of gait, postural instability or levodopa-induced limbs, and cervical dystonia. All patients with homozygous mutation had severe speech impairment. (Table 2) Only two patients underwent DBS. The DBS targets were bilateral Gpi and STN in Patient A and Patient B, respectively. DBS side effects included OFF dyskinesia and speech impairment with freezing of gait in Patient A and Patient B, respectively. (Table 3)
Conclusion: Young-onset PD secondary to PARK7 gene mutation is rare. The clinical features are not widely reported in the literature. In our sample, the patient responded well to dopaminergic medications and had typical and atypical motor features; the nonmotor features included neuropsychiatric symptoms rather than GI symptoms. Our patients showed different responses to DBS regarding benefits and side effects. More research on PD secondary to PARK7 gene mutation must be published to increase understanding of this rare disease.
Table 1
table 2
table 3
References: 1. Klein C, Hattori N, Marras C. MDSGene: Closing Data Gaps in Genotype-Phenotype Correlations of Monogenic Parkinson’s Disease. J Parkinsons Dis. 2018
2. International parkinson and movement disorder society. MDSGene. Mar 2023.
3. Kasten M, Hartmann C, Hampf J, et al. Genotype-Phenotype Relations for the Parkinson’s Disease Genes Parkin, PINK1, DJ1: MDSGene Systematic Review. Mov Disord. 2018
To cite this abstract in AMA style:
H. Alhodaif, Y. Alkhodair, A. Aldakheel, S. Alqahtani, S. Boholegah. Clinical Features of dj1 Gene Mutation Causing Parkinson’s Disease from Single Institution [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/clinical-features-of-dj1-gene-mutation-causing-parkinsons-disease-from-single-institution/. Accessed May 14, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/clinical-features-of-dj1-gene-mutation-causing-parkinsons-disease-from-single-institution/