Session Information
Date: Thursday, June 8, 2017
Session Title: Other
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: To introduce a new practical classification method to assign individuals with Parkinson’s disease (PD) into distinct subtypes using baseline dataset, then to compare neuroimaging, biospecimen markers and disease progression between the subtypes.
Background: PD varies widely in clinical manifestations and prognosis from person to person. Identification of distinct PD subtypes is of great priority to predict progression and develop more efficient personalized care approaches.
Methods: We recruited 421 individuals with de novo early PD from the Parkinson’s Progression Markers Initiative (PPMI). Cluster analysis was performed using a comprehensive dataset at baseline consisting of demographic and genetic information, motor, neuropsychological and other non-motor manifestations. For analysis of longitudinal progression, we created a global composite outcome (GCO) by combining standardized scores of non-motor symptoms, motor symptoms and signs, activities of daily living and global cognition at baseline and the latest follow-up visit.
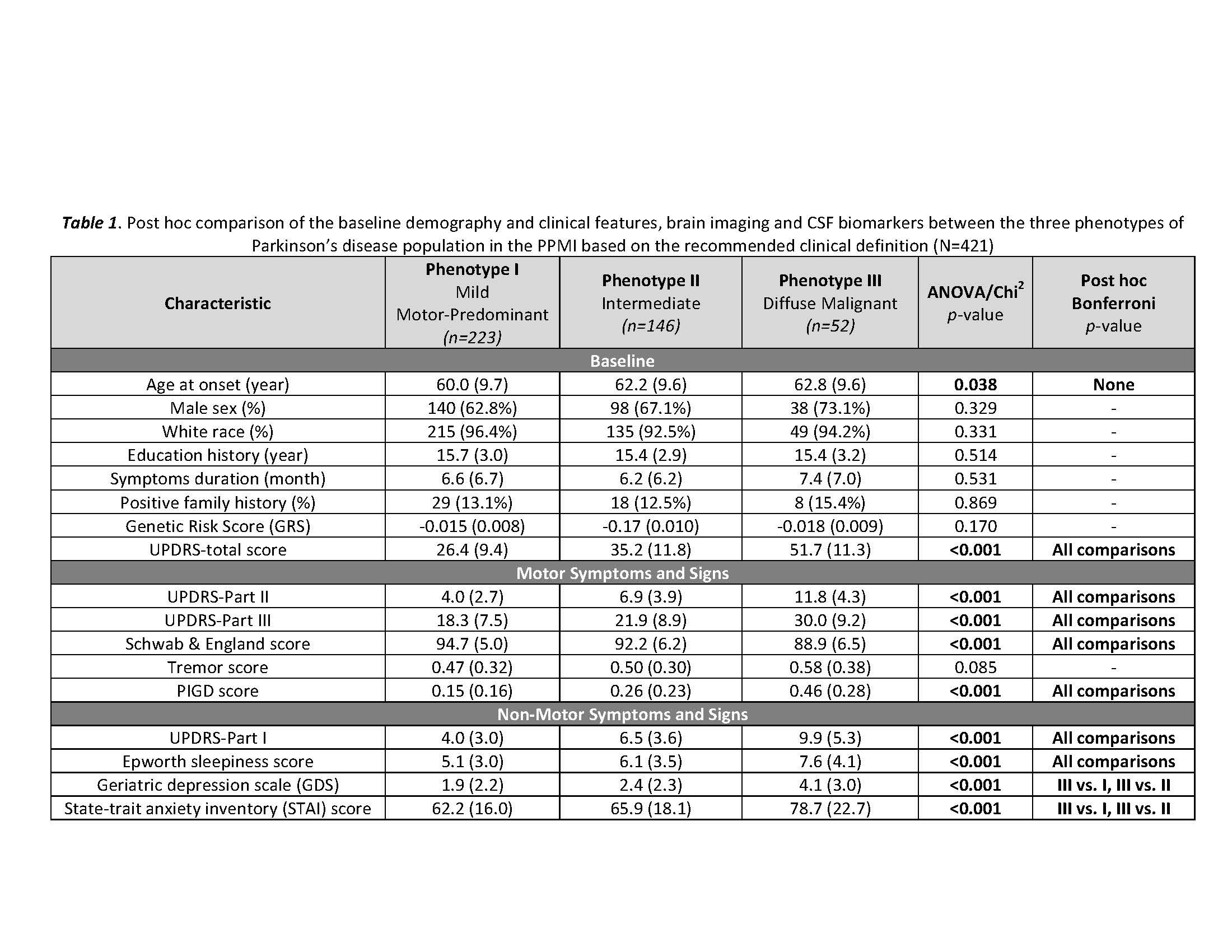
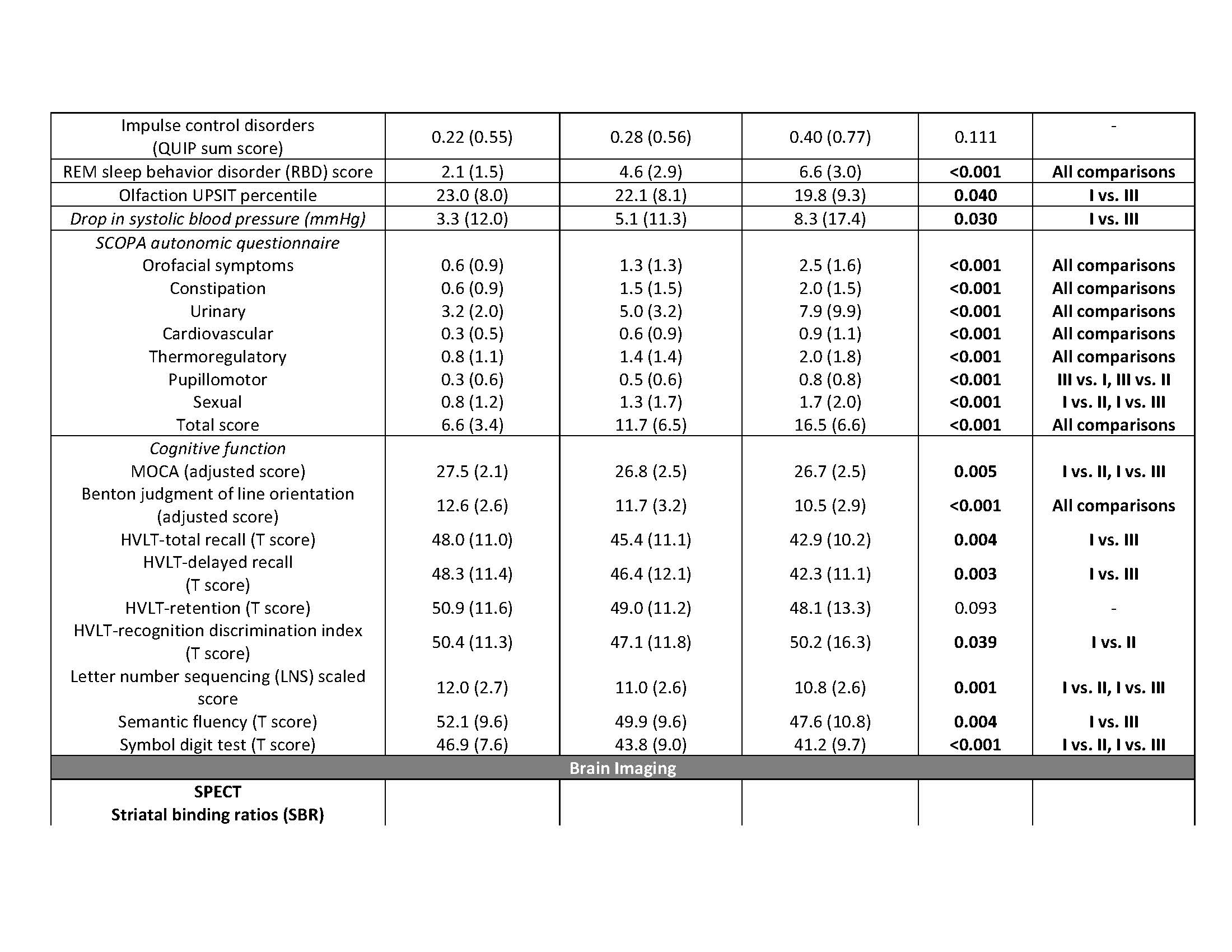
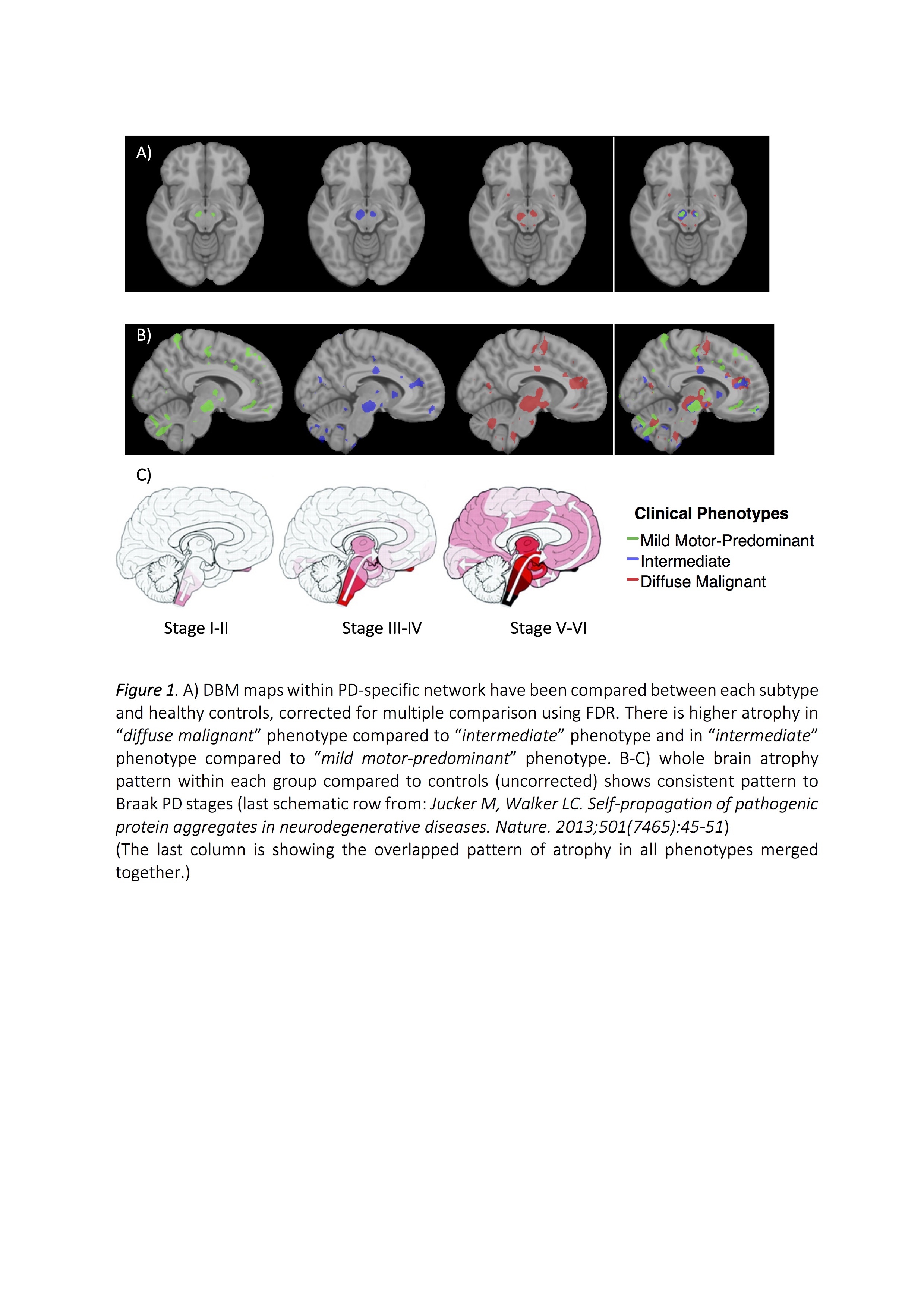
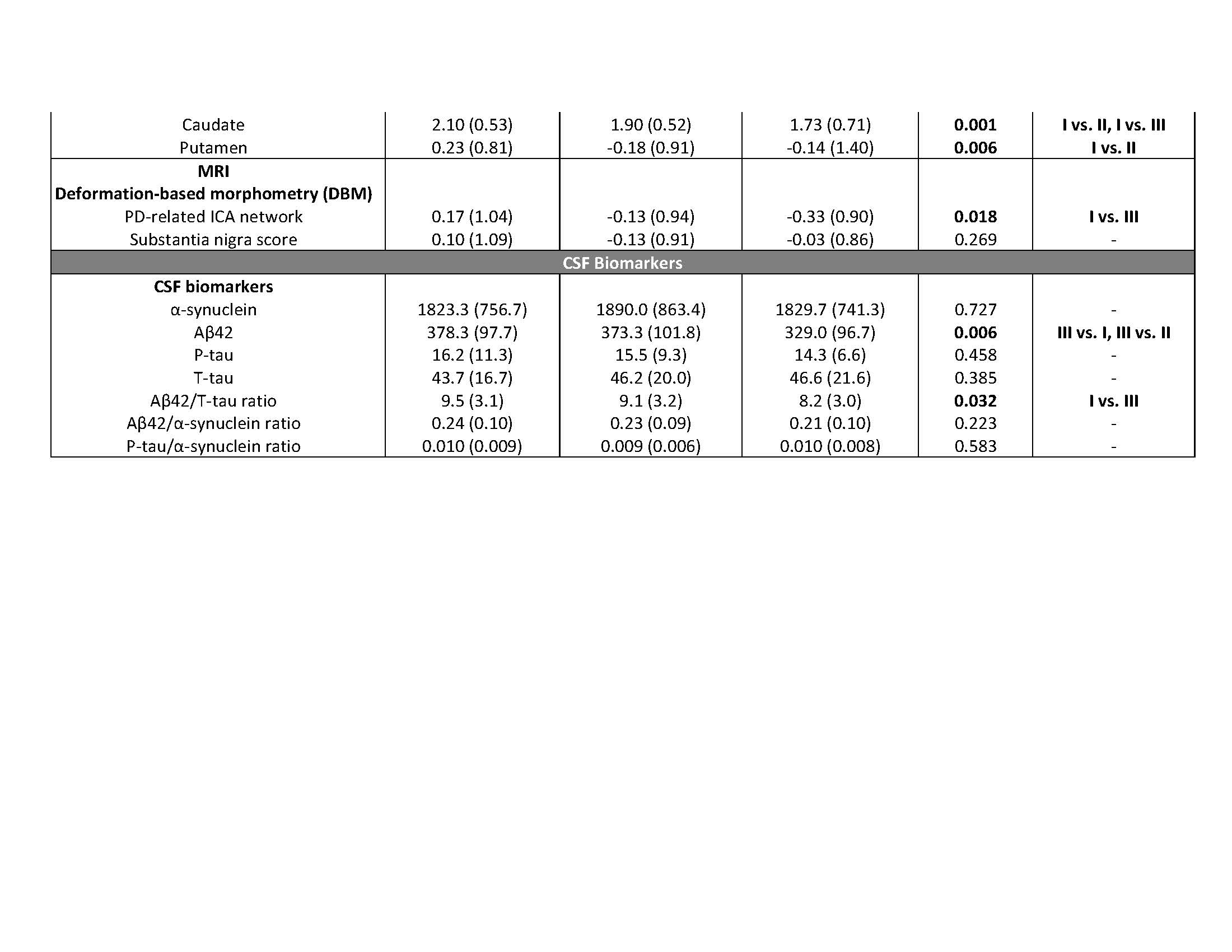
Results: The key classifiers in cluster analysis were motor summary score and three non-motor features (cognitive impairment, RBD and dysautonomia). Based upon this, we assigned individuals into 3 specific subtypes: “mild motor-predominant” (composite motor and all 3 non-motor scores<75th percentile), “diffuse malignant” (composite motor score plus either >1/3 non-motor score>75th percentile, or all three non-motor scores>75th percentile) and “intermediate”. People with “diffuse malignant” PD had the lowest level of CSF amyloid-beta and amyloid-beta/t-tau ratio. A PD-specific brain network in MRI had more atrophy in the “diffuse malignant” subtype, with the “mild motor-predominant” subtype having the least atrophy. Although disease duration and follow-up time were similar, people with “diffuse malignant” PD progressed faster in GCO with greater decline in SPECT measure of dopamine innervation after 2.7 years.
Conclusions: We introduce new clinical criteria for subtyping PD which can be applied in clinical practice using baseline information. Even in the early-stage, patients with “diffuse malignant” subtype demonstrated more profound dopaminergic deficit, increased atrophy in brain network, a more Alzheimer’s disease-like CSF profile and more rapid progression of motor and cognitive deficits.
References: 1. Parkinson Progression Marker I. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011; 95(4): 629-35. 2. Zeighami Y, Ulla M, Iturria-Medina Y, Dadar M, Zhang Y, Larcher KM, et al. Network structure of brain atrophy in de novo Parkinson’s disease. Elife 2015; 4.
To cite this abstract in AMA style:
S.-M. Fereshtehnejad, Y. Zeighami, A. Dagher, R. Postuma. Clinical Criteria for Subtyping Parkinson’s Disease: Differences in imaging and CSF biomarkers and longitudinal progression [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/clinical-criteria-for-subtyping-parkinsons-disease-differences-in-imaging-and-csf-biomarkers-and-longitudinal-progression/. Accessed December 31, 2025.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/clinical-criteria-for-subtyping-parkinsons-disease-differences-in-imaging-and-csf-biomarkers-and-longitudinal-progression/