Category: Parkinson's Disease: Pathophysiology
Objective: Our aim is to investigate whether the circadian cycle (CC) is linked to the patient Parkinson’s disease (PD) profile on a clinical and molecular point of view.
Background: CC abnormalities may contribute to the pathophysiology of neurodegenerative diseases and associate to the PD progression [1]. However, the specific role of CC in PD is still debated [2].
Method: We obtained prospective data on PD phenotype (i.e., UPDRS, Hoehn and Yahr, MoCA), on chronotype by the Morningness Eveningness questionnaire (MEQ), on sleep-wake quality (PDSS, Epworth sleepiness scale – ESS), and on the patient daily motor activity (Activity Index, AI from 6 am to 24 pm) through a wearable sensor embedded in patient smartphones (ToGo App by Mon4t). Moreover, fibroblasts from a subgroup of PD patients (n=5) were also compared to a control group (n=3) for evaluating the CC gene expression (BMAL1, CLOCK, CRY1, PER1, NPAS2) throughout a complete CC and cell count over time [3].
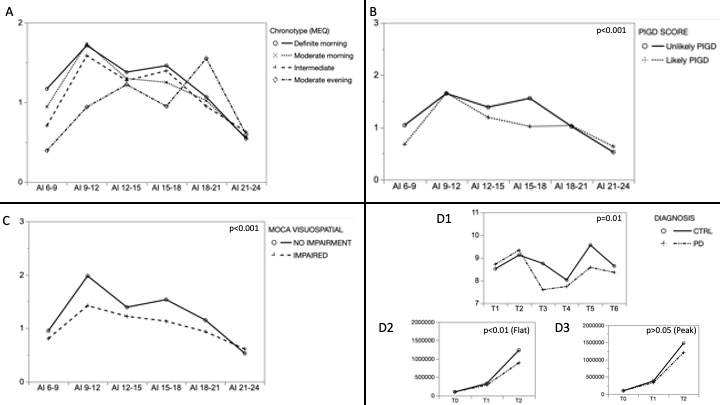
Results: Our population (n= 50, 34% females) presented a mean (±sd) age, disease duration, UPDRS III and H&Y respectively equal to 67±10 years, 7±5 years, 21±9 points, 2±0.5 points. The moderate morning (28, 56%) and the intermediate (16, 32%) were the more frequent chronotypes. The CC of each chronotype was well described by the AI trend, but was statistically identified only by the 6-9 am AI (p<0.01) [Figure 1A]. Chronotypes and AI-CC were not influenced by motor fluctuations and by the PDSS or ESS scores. The Age, the PIGD score (UPDRS III) and the visuospatial MoCA score related to the AI pattern [Figure 1B-C]. The presence of a visuospatial impairment (MoCA visuospatial <5, n=31, 62%) identified a group of subjects with a more flattened CC (MANOVA, p<0.01), independently by the age and by the PIGD score as ascertained by the respective combined models. Corroborating such results, the CC of the BMAL1 gene fibroblast was significantly lower in PD vs controls as well as the cell count who was reduced only in PD with flat BMAL profile (MANOVA, p<0.01) [Figure 1D, 1-3].
Conclusion: Our preliminary study has identified that a worse disease phenotype might feature patients with a different circadian cycle profile. Such results deserve to be further verified on a larger sample with more detailed methodologies. The presence of a dysfunctional (i.e. flat) molecular and clinical circadian cycle might become a promising target for new therapies [4].
References: [1] Obayashi K, Saeki K, Yamagami Y, et al. Circadian activity rhythm in Parkinson’s disease: findings from the PHASE study. Sleep Med. 2021;85:8-14.
[2] Leng Y, Blackwell T, Cawthon PM, et al. Association of Circadian Abnormalities in Older Adults With an Increased Risk of Developing Parkinson Disease. JAMA Neurol. 2020 Oct 1;77(10):1270-1278.
[3] Pacelli C, Rotundo G, Lecce L, et al. Parkin Mutation Affects Clock Gene-Dependent Energy Metabolism. Int J Mol Sci. 2019;20(11):2772.
[4] Willis GL, Freelance CB. The effect of directed photic stimulation of the pineal on experimental Parkinson’s disease. Physiol Behav. 2017;182:1-9.
To cite this abstract in AMA style:
M. Marano, A. Casamassa, M. Rossi, A. Magliozzi, Z. Yekutieli, A. Vescovi, J. Rosati, V. Di Lazzaro. Circadian cycle and disease phenotype in Parkinson’s disease, insights by motion sensors and molecular biology. [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/circadian-cycle-and-disease-phenotype-in-parkinsons-disease-insights-by-motion-sensors-and-molecular-biology/. Accessed October 17, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/circadian-cycle-and-disease-phenotype-in-parkinsons-disease-insights-by-motion-sensors-and-molecular-biology/