Category: Parkinson’s Disease: Clinical Trials
Objective: This interim data analysis preliminarily evaluated the feasibility and effectiveness of protocolized CHM on motor-related daily living of PD patients.
Background: A Chinese national guideline was published to facilitate the use of Chinese herbal medicine (CHM) for Parkinson’s disease (PD). However, its feasibility and effectiveness were not tested, requiring evaluation clinically.
Method: This ongoing pilot add-on, randomised, controlled, pragmatic trial randomised PD patients evenly to receive 32 weeks of add-on CHM prescribed according to their clinical presentations or to continue conventional medication alone. Primary outcome is the part II score of the Movement Disorder Society Sponsored Revision of Unified Parkinson’s Disease Rating Scale (measuring motor-related daily living, higher score indicates more severe presentation). Adverse events are reported based on the Common Terminology Criteria for Adverse Events. Prespecified interim analysis is performed using a linear mixed effect model. (Trial registration: ClinicalTrials.gov, NCT05001217, Date: 8/10/2021)
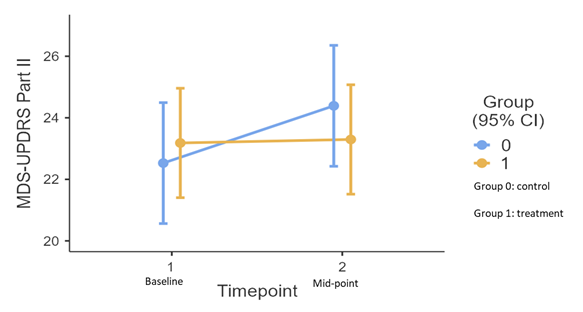
Results: 308 patients were screened, of which 120 were randomized by December 2022 and 80 (treatment: 44 vs control: 36) were included in this analysis. The baseline (treatment vs control) mean (standard deviation) age is 63.1 ± 7.15 vs 62.3 ± 6.86, PD duration is 6.86 ± 4.53 vs 7.14 ± 3.87 years, and the part II score is 23.2 ± 5.94 vs 22.5 ± 5.94, all comparable between groups. A trend of inter-group differences over time was observed in the primary outcome, with the treatment group having a lower score (β: -1.75, CI: -3.52 to 0.026, p=0.057). The test of simple effects show a greater trend of score increment in control (β: 1.86, CI: 0.53 to 3.20, p= 0.007) than in treatment group (β: 0.094. CI: -1.13 to 1.32, p= 0.88) across timepoints (Figure 1). Adverse events include constipation (n=2), non-cardiac chest pain (n=1), falls (n=4, 1 case required hospitalization), dyspepsia (n=3), edema limbs (n=1), and eczema (n=1). They were assessed to be mild to moderate.
Conclusion: Short-term CHM is well-tolerated by participants, showing a trend of between-group improvement in a motor-related daily living outcome.
To cite this abstract in AMA style:
S. Yuen, KK. Chua, L. Zhong, KW. Chan, C. Chan, CP. Ho, KL. Chan, E. Han, K. Pang, ZX. Lin, V. Mok, N. Cheung, AY. Lau, M. Li. Chinese herbal medicine treatment based on subgroup differentiation as adjunct therapy for Parkinson’s disease: interim analysis of a pilot add-on, randomized, controlled, pragmatic clinical trial [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/chinese-herbal-medicine-treatment-based-on-subgroup-differentiation-as-adjunct-therapy-for-parkinsons-disease-interim-analysis-of-a-pilot-add-on-randomized-controlled-pragmatic-clinical-t/. Accessed February 5, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/chinese-herbal-medicine-treatment-based-on-subgroup-differentiation-as-adjunct-therapy-for-parkinsons-disease-interim-analysis-of-a-pilot-add-on-randomized-controlled-pragmatic-clinical-t/