Session Information
Date: Thursday, June 23, 2016
Session Title: Dystonia
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To compare the effects of botulinum toxin type A (BtA) versus placebo in blepharospasm.
Background: This is an update of a Cochrane systematic review. Blepharospasm is a frequent focal dystonia characterized by involuntary eyelid closure due to spasmodic contractions of the orbicularis oculi muscles. Most cases are idiopathic, and severity can range from frequent blinking to persistent eyelid closure with functional blindness. BtA remains the first line therapy.
Methods: Search Methods: Cochrane Movement Disorders Group register, CENTRAL, MEDLINE, EMBASE, reference lists and conference proceedings – last run in December 2015. Selection criteria: double-blind, parallel, randomized, RCTs of BtA in adult patients with blepharospasm. Data collection: two independent authors assessed records, selected included studies, extracted data and evaluated the risk of bias. Disagreements were solved by consensus or by a third element.
Results: We included 2 studies (n=252) with a moderate quality overall. 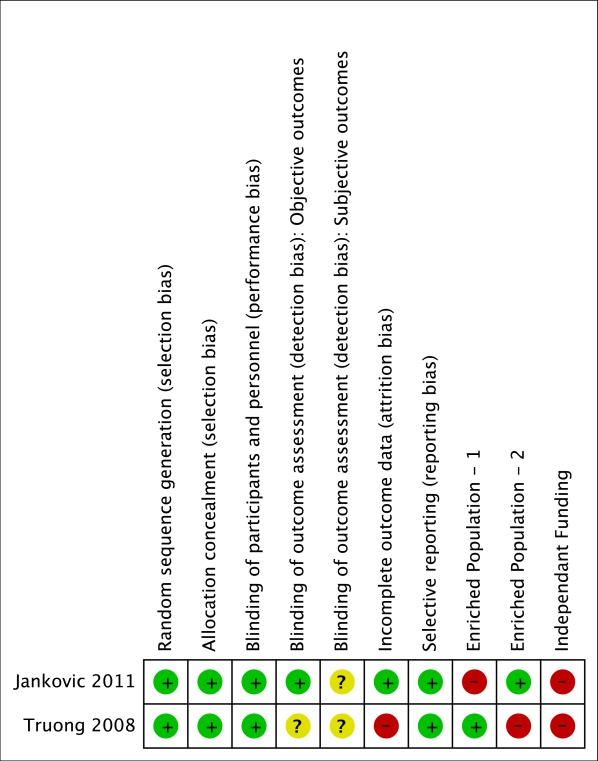 One study excluded clinical forms known to respond poorly to BtA administration (such as eyelid apraxia), and the other enrolled only patients with a documented stable therapeutic response to BtA. In both trials, patients received one treatment per eye, with a wide range of employed doses. BtA was associated with a reduced blepharospasm severity (SMD -0.86, 95%CI: -1.17 to -0,55) and frequency (SMD -1, 95%CI: -1.87 to -0,13) at 3-6 weeks after injection. Both patients (RR 3.76, 95%CI: 2.31 to 6.12) and investigators (RR 3.07, 95%CI: 2.00 to 4.71) assessments of overall improvement at final visit largely favoured BtA over placebo. However, the intervention was associated with increased eyelid ptosis (RR 5.77, 95%CI 1.82 to 18.34), visual complaints (RR 5.73, 95%CI 1.79 to 18.36) and, non-significantly, lacrimation (RR 2.04, 95%CI 0.46 to 9.13) and xerophthalmia (RR 1.83, 95%CI 0.69 to 4.86). Statistical heterogeneity between study results was low for most outcomes.
One study excluded clinical forms known to respond poorly to BtA administration (such as eyelid apraxia), and the other enrolled only patients with a documented stable therapeutic response to BtA. In both trials, patients received one treatment per eye, with a wide range of employed doses. BtA was associated with a reduced blepharospasm severity (SMD -0.86, 95%CI: -1.17 to -0,55) and frequency (SMD -1, 95%CI: -1.87 to -0,13) at 3-6 weeks after injection. Both patients (RR 3.76, 95%CI: 2.31 to 6.12) and investigators (RR 3.07, 95%CI: 2.00 to 4.71) assessments of overall improvement at final visit largely favoured BtA over placebo. However, the intervention was associated with increased eyelid ptosis (RR 5.77, 95%CI 1.82 to 18.34), visual complaints (RR 5.73, 95%CI 1.79 to 18.36) and, non-significantly, lacrimation (RR 2.04, 95%CI 0.46 to 9.13) and xerophthalmia (RR 1.83, 95%CI 0.69 to 4.86). Statistical heterogeneity between study results was low for most outcomes.
Conclusions: High quality evidence suggests BtA is an efficacious therapeutic approach to blepharospasm, and despite the increased risk of side effects, patients still consider it highly beneficial. 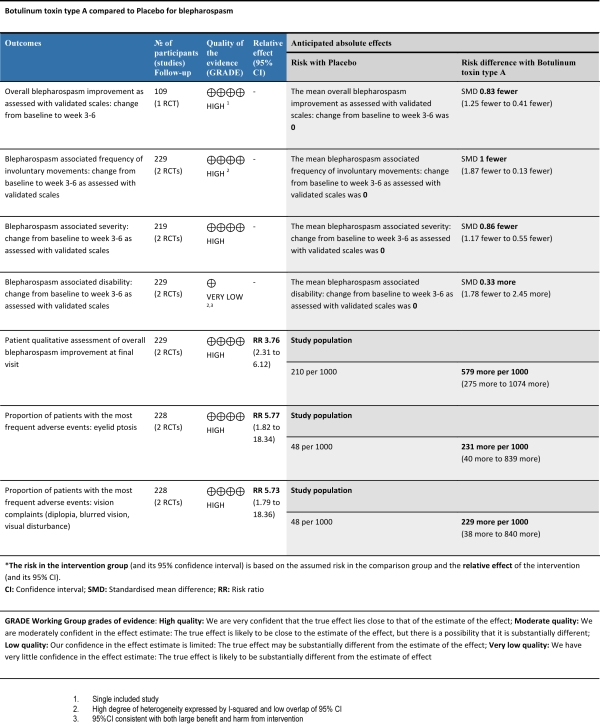 Though treatment effect has been well stablished, future trials should still perform safety analysis and explore technical factors such as doses, formulations, optimum treatment intervals and the effect of repeated injections.
Though treatment effect has been well stablished, future trials should still perform safety analysis and explore technical factors such as doses, formulations, optimum treatment intervals and the effect of repeated injections.
To cite this abstract in AMA style:
R.E. Marques, G.S. Duarte, F.B. Rodrigues, M. Castelão, J.J. Ferreira, P. Moore, J. Costa. Botulinum toxin type A therapy for blepharospasm – Update of a Cochrane systematic review and meta-analysis [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/botulinum-toxin-type-a-therapy-for-blepharospasm-update-of-a-cochrane-systematic-review-and-meta-analysis/. Accessed April 20, 2025.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/botulinum-toxin-type-a-therapy-for-blepharospasm-update-of-a-cochrane-systematic-review-and-meta-analysis/
