Session Information
Date: Thursday, June 23, 2016
Session Title: Dystonia
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To update a Cochrane review comparing botulinum neurotoxin type A (BtA) to type B (BtB) in adults with cervical dystonia (CD).
Background: This is an update of a previous Cochrane systematic review (Costa 2003). CD is a disabling disorder characterized by involuntary head posturing and is the most frequent type of dystonia. BtA remains the gold-standard for its treatment but BtB is also an approved indication. While both have been shown to be effective and safe versus placebo, they have not been systematically compared to each other.
Methods: Search Methods: Cochrane Movement Disorders Group trials register, CENTRAL, MEDLINE, EMBASE, as well as reference lists of articles and conference proceedings, last run in December 2015. Selection criteria: double-blind, parallel, randomized, placebo-controlled trials of BtB in adult patients with cervical dystonia. Data collection: two independent authors assessed records, selected included studies, extracted data and evaluated the risk of bias. Disagreements were solved by consensus or by a third element.
Results: We included 3 studies (n=269) with a medium-to-low risk of bias.  BtA and BtB did not perform differently on validated clinical scales (MD -1.44, 95%CI -3.68 to 0.70), nor on disability (MD -0.22, 95%CI -1.21 to 0.76) or severity (MD -0.31, 95%CI -1.27 to 0.65) subscales. BtB-treated patients scored 0.99 fewer points on pain subscales (95%CI -1.85 to -0.12). Subjective patient and clinician assessments overlooked differences. Adverse event proportions were comparable (RR 0.72, 95%CI 0.51 to 1.00), but BtA-treated patients had 0.49 (95%CI 0.32 to 0.75) and 0.42 (95%CI 0.31 to 0.57) times fewer dysphagia and sore throat/dry mouth events, respectively.
BtA and BtB did not perform differently on validated clinical scales (MD -1.44, 95%CI -3.68 to 0.70), nor on disability (MD -0.22, 95%CI -1.21 to 0.76) or severity (MD -0.31, 95%CI -1.27 to 0.65) subscales. BtB-treated patients scored 0.99 fewer points on pain subscales (95%CI -1.85 to -0.12). Subjective patient and clinician assessments overlooked differences. Adverse event proportions were comparable (RR 0.72, 95%CI 0.51 to 1.00), but BtA-treated patients had 0.49 (95%CI 0.32 to 0.75) and 0.42 (95%CI 0.31 to 0.57) times fewer dysphagia and sore throat/dry mouth events, respectively.
Conclusions: Low-to-moderate quality evidence showed similar safety and efficacy profiles between BtA and BtB with the exception that BtA-treated patients had fewer dysphagia and dry mouth events.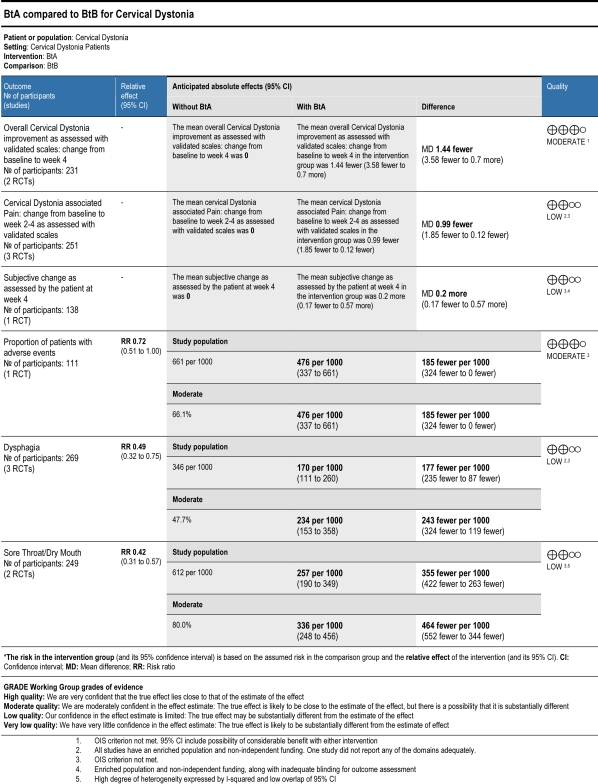 The findings of this update provide the best evidence currently available, but also reveal areas of uncertainty that should be addressed in future research. Further studies should consider recruiting larger samples and address questions such as the effects of multiple treatment cycles, the role of immunogenicity resistance, the impact of Bt on quality of life, and subgroup analysis of treatment naïve patients versus subjects previously treated with BtA.
The findings of this update provide the best evidence currently available, but also reveal areas of uncertainty that should be addressed in future research. Further studies should consider recruiting larger samples and address questions such as the effects of multiple treatment cycles, the role of immunogenicity resistance, the impact of Bt on quality of life, and subgroup analysis of treatment naïve patients versus subjects previously treated with BtA.
To cite this abstract in AMA style:
M. Castelão, G.S. Duarte, F.B. Rodrigues, R.E. Marques, J.J. Ferreira, P. Moore, J. Costa. Botulinum neurotoxins type A versus B for cervical dystonia in adults – 2016 Cochrane movement disorders group systematic review update [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/botulinum-neurotoxins-type-a-versus-b-for-cervical-dystonia-in-adults-2016-cochrane-movement-disorders-group-systematic-review-update/. Accessed July 8, 2025.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/botulinum-neurotoxins-type-a-versus-b-for-cervical-dystonia-in-adults-2016-cochrane-movement-disorders-group-systematic-review-update/
