Category: Parkinson's Disease: Pathophysiology
Objective: Here, we aim to model body- and brain-first PD by injecting pathology in the gut and amygdala of old wild-type rats, respectively.
Background: Parkinson’s disease (PD) is caused by pathology that can propagate in a stereotypical pattern along the brain-body axis, affecting multiple organs. PD patients display highly heterogeneous symptoms and variable involvement of different neuronal systems during the early disease stages, complicating diagnosis. Emerging data from post-mortem and imaging studies suggest that disease heterogeneity could be explained by variable disease onset sites (brain vs. body) and the brain connectome, also known as the SOC model. This model proposed two PD subtypes: (1) a brain-first type, where pathology initially appears in a single hemisphere in the brain, causing more asymmetric parkinsonism and secondary spreading to the peripheral autonomic nervous system (ANS); and (2) a body-first type, where the pathology originates in the peripheral ANS (usually the gut), after which it spreads to the brain bilaterally, causing more symmetric parkinsonism. Animal models are important to gain mechanistic insight into prodromal disease subtype differences, and to explore and test early subtype-specific biomarkers and disease-modifying treatments.
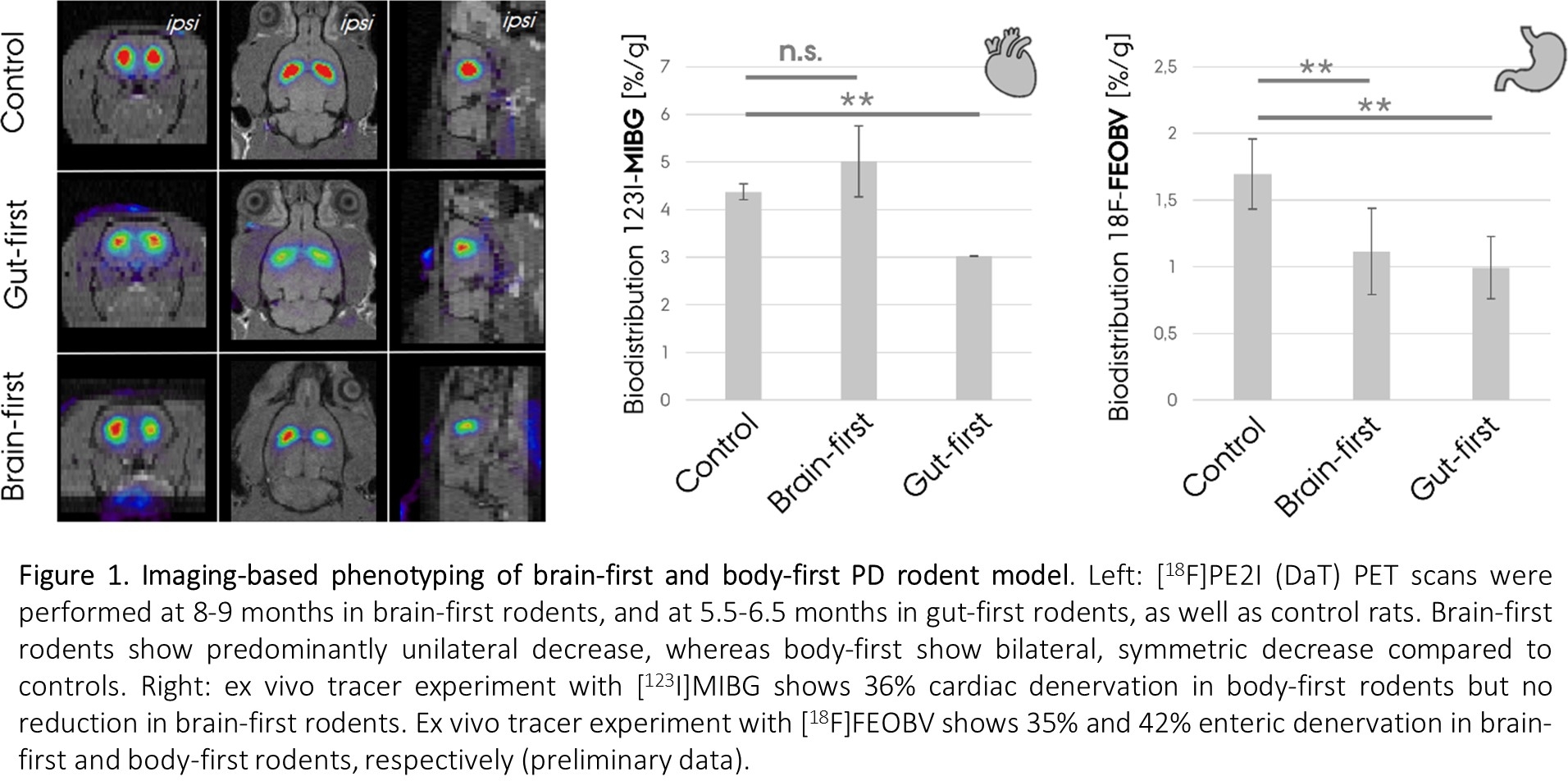
Method: Characterization of both models is performed using immunostainings against pathology, [18F]PE2I (DaT) PET scans, ex vivo [123I]MIBG (cardiac) and [18F]FEOBV (enteric) tracer readouts and symptom scoring.
Results: Our gut-first model mimics human body-first PD with symmetric pathology in the brain, ultimately leading to bilateral dopaminergic neurodegeneration (Fig. 1). Additionally, we observe cardiac and enteric denervation, and pathology in peripheral tissues, including the skin. Our brain-first model appears to mimic brain-first human PD, with unilateral pathology at early stages that evolve into predominant unilateral neurodegeneration. In the brain-first rodents, we did not observe cardiac denervation, similar to human brain-first PD (Fig. 1).
Conclusion: Our two complementary animal models are the first direct attempt to recapitulate human PD subtypes, with a comprehensive approach including several PD features (old age, peripheral pathology etc.). This study also supports the SOC model and provides compelling mechanistic insight into why PD is an asymmetric disorder in some patients, and symmetric in others, potentially solving this hitherto unexplained mystery.
To cite this abstract in AMA style:
N. Vanden Berge, M. Pedersen, I. Klæstrup, J. Jacobsen, M. Simonsen, A. Alstrup, M. Romero-Ramos, P. Borghammer. Body-first and brain-first PD animal models mimic human PD subtypes [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/body-first-and-brain-first-pd-animal-models-mimic-human-pd-subtypes/. Accessed December 14, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/body-first-and-brain-first-pd-animal-models-mimic-human-pd-subtypes/