Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate the safety and feasibility of MR guided focused ultrasound (MRgFUS) blood-brain barrier (BBB) opening in the cortex or the striatum in Parkinson´s disease (PD) patients with cognitive impairment and putative changes in cognition and PET.
Background: Dementia in PD (PDD), reaches 80% 20 years after diagnosis. In PDD, there is high combination of mixed Alzheimer´s (AD) and PD pathology and current treatments provide very modest benefit. BBB penetration is a major restriction for many putative therapeutic agents. Low frequency MR guided focused ultrasound MRgFUS in combination with intravenous microbubble administration leads to focal temporary BBB opening, which makes it a putative tool for drug delivery. In the mice amyloid AD model, BBB opening produced significant reduction in brain plaques.
Method: Open, proof-of-concept, safety and feasibility pilot study of focal BBB opening in PDD. Six patients underwent MRgFUS procedure to open the BBB twice, separated by at least two weeks. The right parietooccipitotemporal cortex was targeted in the first 5 patients and the right striatum in the sixth case. Immediately, 1 and 7 days after the procedure, brain MR with gadolinium was taken to detect enhancement indicating BBB opening and closure. Amyloid and fluordeoxyglucose PET were performed before treatment and 3 weeks after the second BBB opening session in the first five patients and amyloid and fluorodopa PET before treatment and 3 and 6 months after the second session in patient #6 .
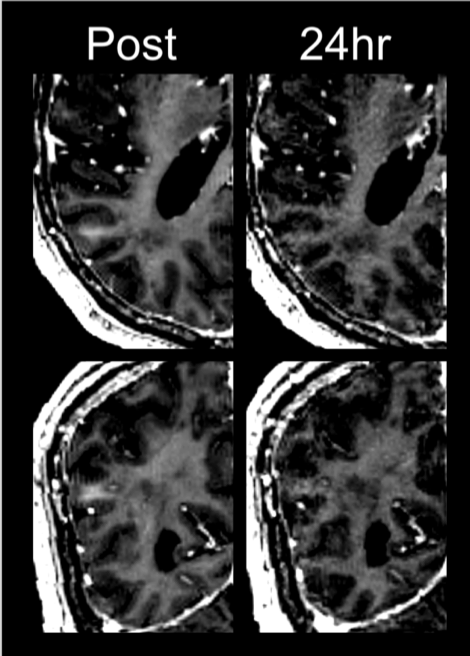
Results: BBB was opened satisfactorily after treatments and was closed in all cases by day 7. There were no major side effects and mild improvement in several cognitive domains was observed in the first 5 patients evaluated 3 weeks after the second treatment. Discrete round hypointensities on MR-SWAN sequences (sonicated region) were seen immediately after treatment. These disappeared progressively except for one patient who still showed some minor abnormalities after 2 months. No clinical manifestation associated with this signal was encountered. There were no major changes from pre to post treatment in the PET studies.
Conclusion: This study suggests that MRgFUS-BBB opening in the cortex and the striatum in PDD is safe, reversible and can be done repeatedly. Therapeutic trials combining parenteral drug delivery and BBB opening can be envisaged to treat neurodegenerative dementia.
To cite this abstract in AMA style:
C. Gasca-Salas, J. Pineda-Pardo, B. Fernández-Rodríguez, R. Rodríguez-Rojas, F. Hernández, I. Obeso, M. del Álamo, P. Guida, D. Mata-Marin, R. Martínez-Fernández, C. Ordás-Bandera, G. Foffani, I. Rachmilevitch, J. Obeso. Blood-Brain Barrier Opening with Focused Ultrasound in Parkinson´s Disease with Cognitive Impairment: A Safety and Feasibility Study [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/blood-brain-barrier-opening-with-focused-ultrasound-in-parkinsons-disease-with-cognitive-impairment-a-safety-and-feasibility-study/. Accessed December 17, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/blood-brain-barrier-opening-with-focused-ultrasound-in-parkinsons-disease-with-cognitive-impairment-a-safety-and-feasibility-study/