Category: Drug-Induced Movement Disorders
Objective: Tardive dyskinesia (TD) describes various movement disorders developing after treatment with dopamine receptor blocking agents (DRBA), including stereotypy, akathisia, dystonia, tremor, tics and chorea. TD develops after at least three months of DRBA exposure and persists for at least one month after treatment discontinuation [1].
Background: Diagnosis is based on patient history of DRBA exposure, clinical presentation and differential diagnosis exclusion. Because little is known about 18-FDG PET-CT imaging in TD, we present the case of a TD patient with imaging abnormalities.
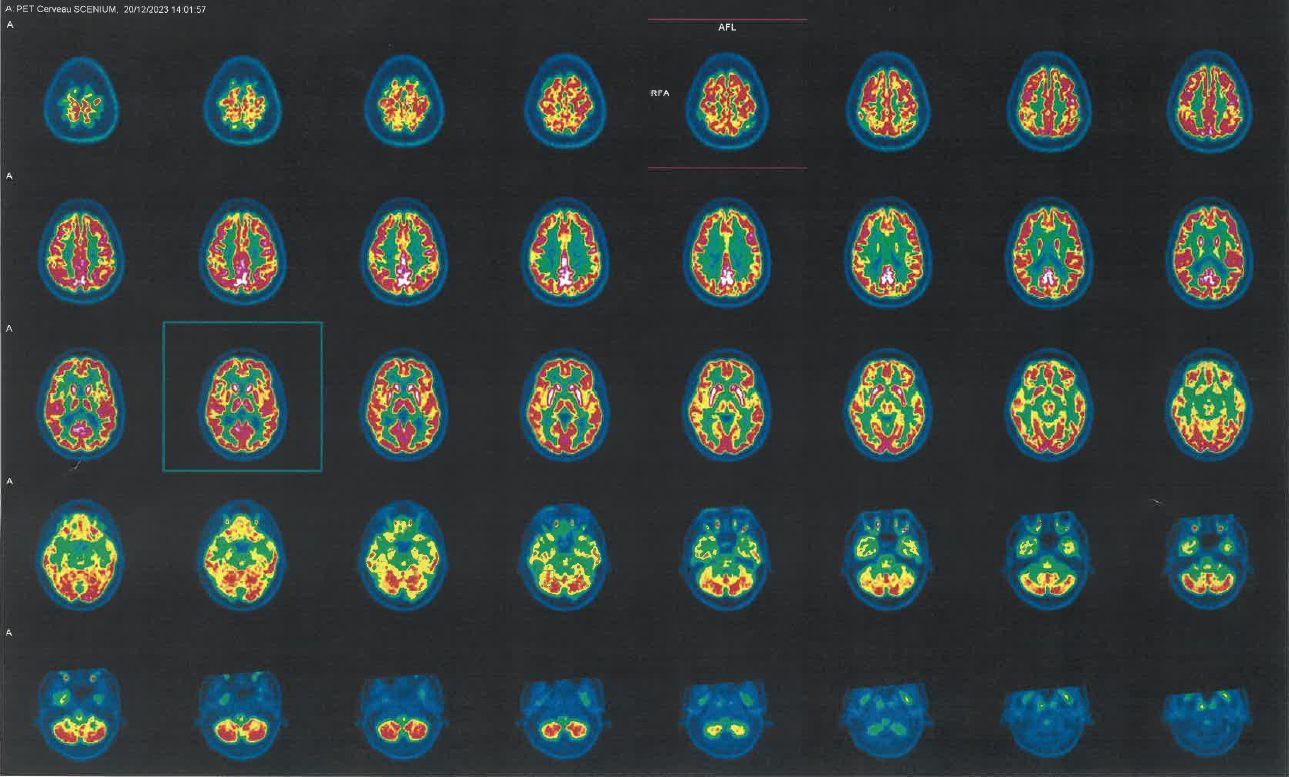
Method: We report the case of a 50 year-old woman presenting with progressive involuntary movements of the face, neck and lower limbs following treatment with ARIPIPRAZOLE for depressive syndrome. Because of atypical clinical presentation with worsening a few months after ARIPIPRAZOLE discontinuation and a major hypermetabolism of bilateral striata and of the posterior cingulum on 18 FDG PET-CT (Figure 1), we investigated for auto-immune encephalitis. This was ruled out by negative auto-antibodies on cerebrospinal fluid and lack of clinical response after three courses of intravenous immunoglobulins and lead us to conclude to TD. Tetrabenazine however showed partial efficacy and patient is currently in eligibility evaluation for deep brain stimulation of thee globus pallidus internus.
Results: Review of the literature did not reveal bistriatal hypermetabolism to be described in TD, but in coherence with PET-CT abnormalities which are well described in some hyperkinetic movement disorders. Chorea in Huntington’s disease or tics are associated with striatal hypometabolism, whereas dystonia can be associated with hypermetabolism in the striatum [2, 3]. This data suggests striatum plays a key role in hyperkinetic movement disorders. However, literature is lacking concerning 18-FDG PET-CT imaging in TD. One study revealed vulnerability to development of TD in patients with schizophrenia was associated with a specific 18-FDG PET-CT pattern, including hypermetabolism in the caudate nucleus [4]. To our knowledge, our case is the first description of striatal hypermetabolism in TD. Nuclear imaging is currently not used in TD diagnosis, but describing a typical pattern could aid in positive diagnosis and avoid encephalitis check-up.
Conclusion: We here report the first case in our knowledge of TD associated with intense striatal hypermetabolism in PET-CT.
PET-CT imaging of our patient
References: 1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed., Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000. p 803–805
2. Timmers ER, Klamer MR, Marapin RS, Lammertsma AA, de Jong BM, Dierckx RAJO, et al. [18F]FDG PET in conditions associated with hyperkinetic movement disorders and ataxia: a systematic review. Eur J Nucl Med Mol Imaging. 2023;50(7):1954–73.
3. Berti V, Pupi A, Mosconi L. PET/CT in diagnosis of movement disorders. Annals of the New York Academy of Sciences. 2011;1228(1):93–108.
4. Szymanski S, Gur RC, Gallacher F, Mozley LH, Gur RE. Vulnerability to Tardive Dyskinesia Development in Schizophrenia: An FDG-PET Study of Cerebral Metabolism. Neuropsychopharmacol. 1996 Dec;15(6):567–75.
To cite this abstract in AMA style:
C. Héraud, C. Alecu, C. Giordana. Bistriatal hypermetabolism on 18-FDG PET-CT in tardive dyskinesia [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/bistriatal-hypermetabolism-on-18-fdg-pet-ct-in-tardive-dyskinesia/. Accessed January 1, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/bistriatal-hypermetabolism-on-18-fdg-pet-ct-in-tardive-dyskinesia/