Category: Surgical Therapy: Parkinson's Disease
Objective: We present a case of a 59-year-old PD patient with severe intolerance to the standard anti-Parkinsonian drugs (APDs) including levodopa (L-DOPA), MAO-B inhibitors, and higher doses of dopamine agonists, treated with bilateral subthalamic nucleus (STN) deep brain stimulation (DBS).
Background: Deep brain stimulation (DBS) is an effective treatment option for patients with advanced Parkinson disease (PD) and motor complications. The “good” candidates demonstrate an excellent response to all APDs and have at least a 30% motor improvement (UPDRS-III) during the L-DOPA challenge.
Method: The patient had his first symptoms before 11 years and a PD diagnosis before 9 years. He had a severe intolerance to MAO-B inhibitors (Rasagiline, Selegiline), dopamine agonists (pramipexole), and L-DOPA. The only tolerable APD was Amantadine sulfate in a minimal dose of 100mg/daily. He had a Hoehn & Yahr Stage 3 disease, without any cognitive and psychiatric manifestations. During the L-DOPA challenge, there was a good response with a 62% improvement – UPDRS-III:24 in OFF-medication to UPDRS-III:9 in ON-medication state. Although, severe side effects were noted – dizziness, nausea, blurred vision, and abdominal discomfort. After a thorough pre-surgical screening, a decision for bilateral STN DBS was taken due to the intolerance to APDs including severe side-effects to L-DOPA.
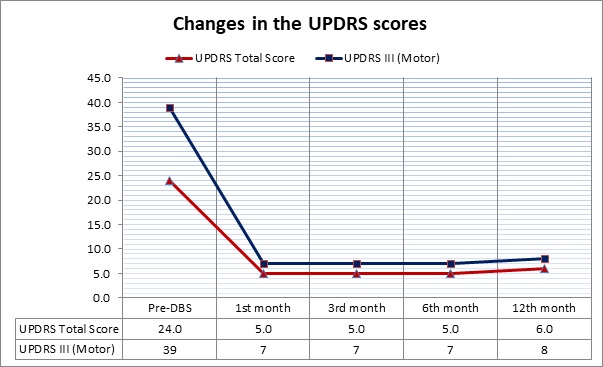
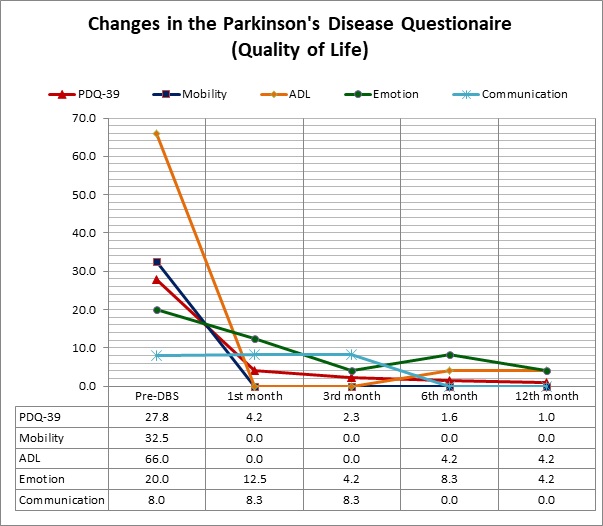
Results: The patient had surgery with simultaneous implantation of bilateral STN leads and a pulse generator without any complications. Intraoperative microstimulation showed a significant reduction in motor symptoms bilaterally. The initial programming session was 4 weeks after the surgery, as we used a monopolar configuration with a constant-current DBS stimulation bilaterally (Left-STN:3.80mA; Right-STN:3.30mA; Frequency:130Hz; Pulse width: 60 μs). The results were remarkable with a UPDRS-III:5 during DBS-ON stimulation. During the 1 year follow-up, no change in the stimulation parameters was needed, as the UPDRS scores remained unchanged (Figure 1). The PDQ-39 scale revealed a constant, significant improvement in all domains during this period (Figure 2). There were no surgical or stimulation-induced side-effects.
Conclusion: Deep brain stimulation is a therapeutic option with substantial benefit in patients with intolerance to standard anti-parkinsonian drugs, including L-DOPA resistance.
To cite this abstract in AMA style:
M. Tsalta-Mladenov, V. Dimitrova, M. Moynov, S. Andonova, Y. Enchev. BILATERAL SUBTHALAMIC NUCLEUS DEEP BRAIN STIMULATION IN A PATIENT WITH PARKINSON DISEASE AND INTOLERANCE TO STANDARD ANTIPARKINSONIAN DRUGS – A CASE REPORT [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/bilateral-subthalamic-nucleus-deep-brain-stimulation-in-a-patient-with-parkinson-disease-and-intolerance-to-standard-antiparkinsonian-drugs-a-case-report/. Accessed December 22, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/bilateral-subthalamic-nucleus-deep-brain-stimulation-in-a-patient-with-parkinson-disease-and-intolerance-to-standard-antiparkinsonian-drugs-a-case-report/