Objective: The aim of this analysis was to identify the baseline variables that differ between patients who completed Study CTH-301 and those who discontinued during the dose optimisation or long-term maintenance phase due to either lack of efficacy or adverse events (AEs).
Background: The Phase 3 Study CTH-301 evaluated the long-term safety/tolerability and efficacy of apomorphine sublingual film (SL-APO) for treating OFF-episodes in Parkinson’s disease patients with motor fluctuations. Data from this study were used to build a model to identify baseline variables that differ between patients who completed the study and those who discontinued, and that might therefore influence SL-APO retention.
Method: Baseline variables were ranked based on their correlation (Chi-square) with the discrete target variable (completers vs non-completers due to lack of efficacy or AEs). A logistic regression classification algorithm with LASSO regularisation was used to select the final variables.
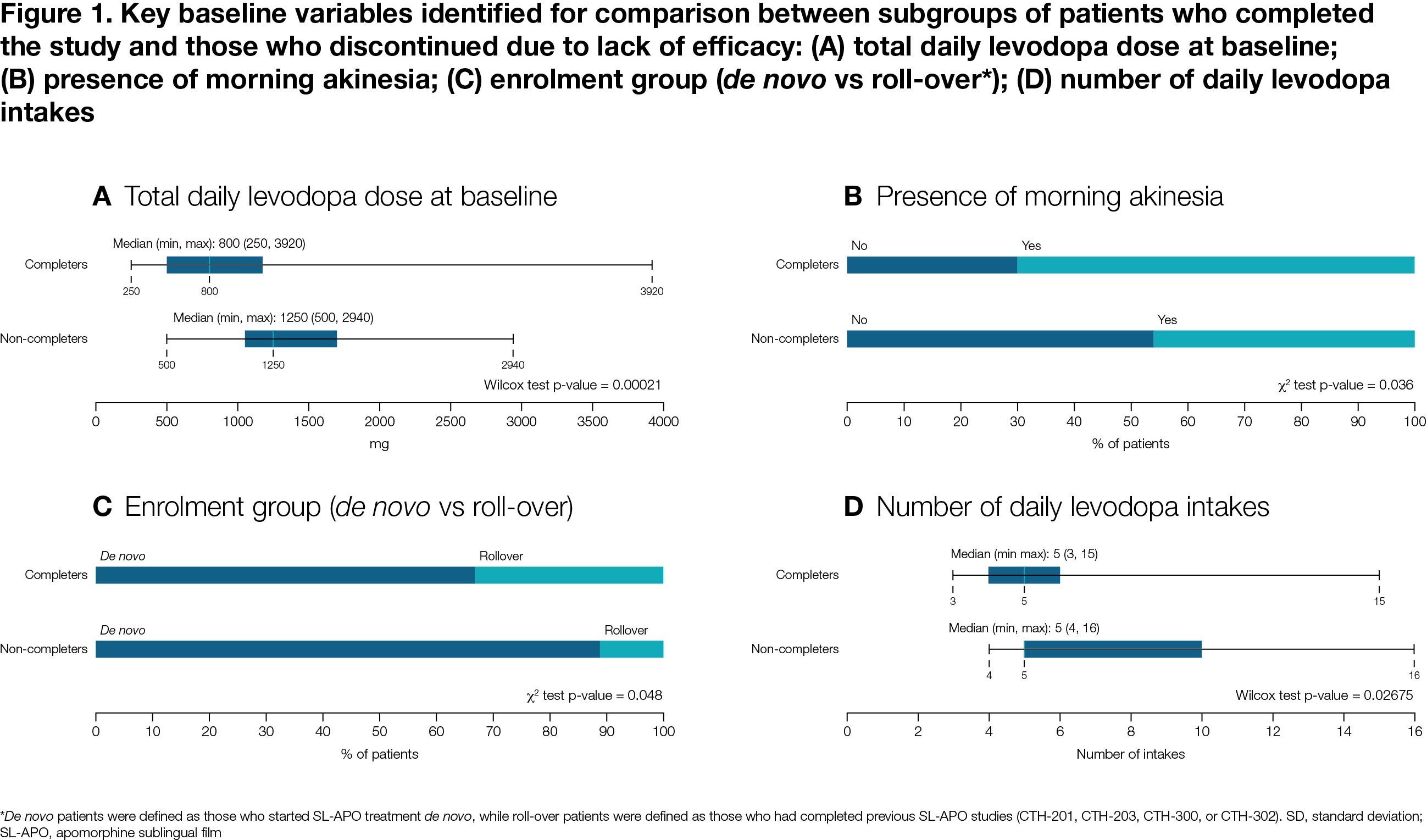
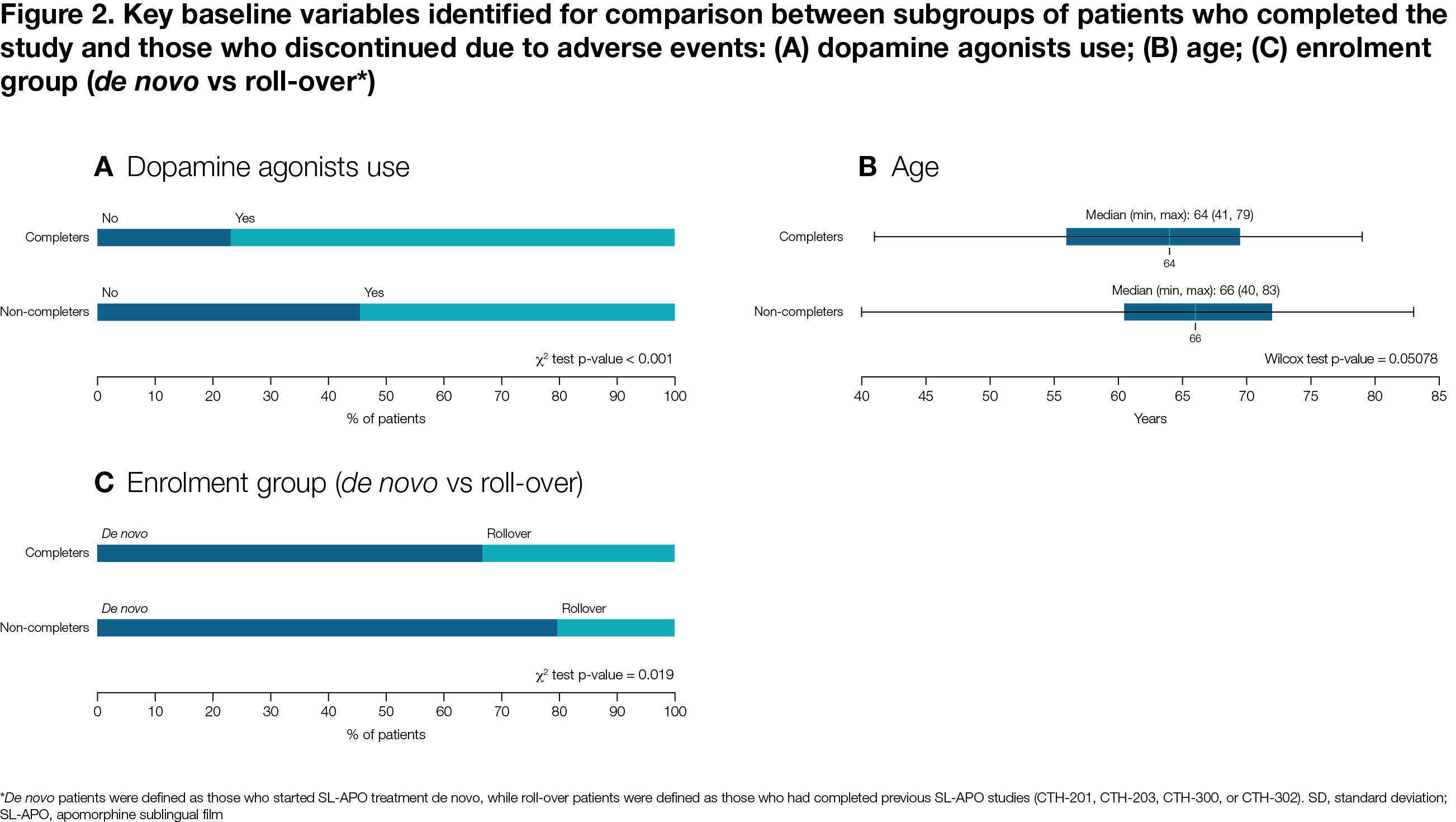
Results: Of 496 patients, 120 completed the study, 26 discontinued due to lack of efficacy, 167 due to AEs, and 183 due to other reasons. Median time on SL-APO was 204 days. Compared to completers, non-completers due to lack of efficacy demonstrated a higher levodopa total daily dose (p<0.001), lower rate of morning akinesia (p=0.036), higher rate of de novo enrolment (no previous SL-APO exposure; p=0.048) and higher number of levodopa intakes (p=0.027) [Figure 1]. Compared to completers, non-completers due to AEs had a lower rate of concomitant dopamine agonists use (p<0.001) and higher rate of de novo enrolment (p=0.019) [Figure 2].
Conclusion: Levodopa dose and intakes, morning akinesia, previous SL-APO exposure and concomitant dopamine agonists use might influence retention. These results may help identify patients more likely to remain on SL-APO over the long term.
Figure 1
Figure 2
To cite this abstract in AMA style:
J. Kassubek, J. Schwarz, L. López Manzanares, M. Fonseca, C. Denecke Muhr. Baseline Variables Associated with Apomorphine Sublingual Film Retention [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/baseline-variables-associated-with-apomorphine-sublingual-film-retention/. Accessed January 18, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/baseline-variables-associated-with-apomorphine-sublingual-film-retention/