Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate baseline CSF and blood-based biomarkers of pathology in early-stage Parkinson’s Disease (PD) participants in the phase 2 clinical trial PASADENA.
Background: Observational and interventional studies in PD require an understanding of how biomarkers of α-synuclein, neurodegeneration and neuroinflammation are interrelated and linked to clinical outcomes.
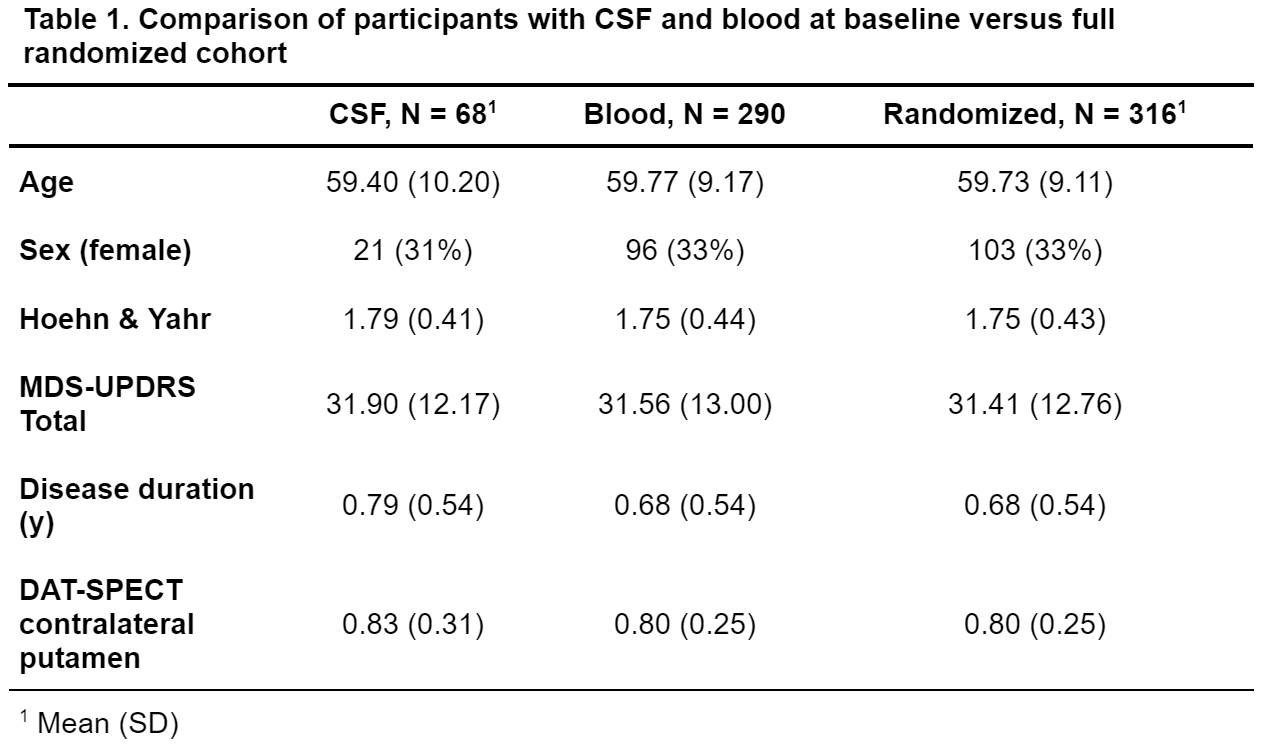
Method: 68 out of 316 randomized participants consented to baseline lumbar puncture. CSF samples were submitted to α-synuclein seed amplification assay (SAA) and NeuroToolkit (NTK) analysis (Abeta40, Abeta42, Interleukin 6, Neurofilament Light Chain [NFL], soluble TREM2 [sTREM2], total α-Synuclein, Tau, phosphorylated Tau [pTau] and YKL-40 were tested). 290 baseline blood samples underwent NTK analysis (7 biomarkers; total α-Synuclein and pTau not available). Demographic and clinical measures were not different between CSF/blood and full randomized cohorts [table 1].
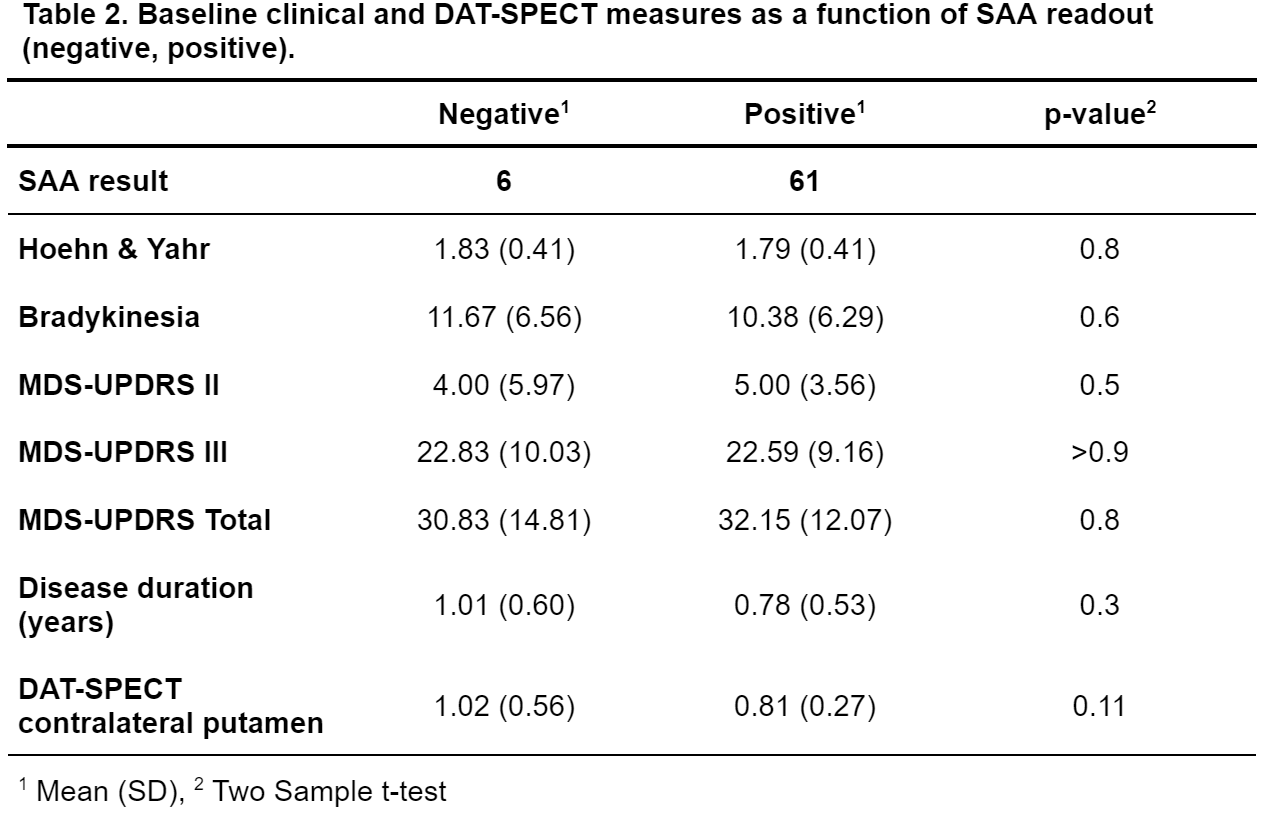
Results: 61/68 baseline PASADENA CSF samples amplified above threshold (positive), 6 remained negative (1 inconclusive). There were no significant SAA-related differences regarding clinical status (MDS-UPDRS II, III, Total and Hoehn & Yahr stage; all p>0.7, [table2]).
Kinetic parameters (time to threshold, time to 50% max fluorescence, max fluorescence) of positive SAA samples did not correlate with clinical endpoints: all p>.66 for MDS-UPDRS II, III and Total scores (Spearman correlation used throughout).
CSF NTK biomarkers were not correlated with MDS-UPDRS II, III or Total (n.b. NFL x MDS-UPDRS III, rho=.28, p=0.025). In the blood NTK sample, Abeta40 (rho=.14, p=.021), sTREM2 (rho=.17, p=0.005) and YKL-40 (rho=.13, p=0.033) were weakly positively correlated with MDS-UPDRS III, Abeta40 (rho=.12, p=.044) and sTREM2 (rho=.17, p=0.006) also with MDS-UPDRS Total.
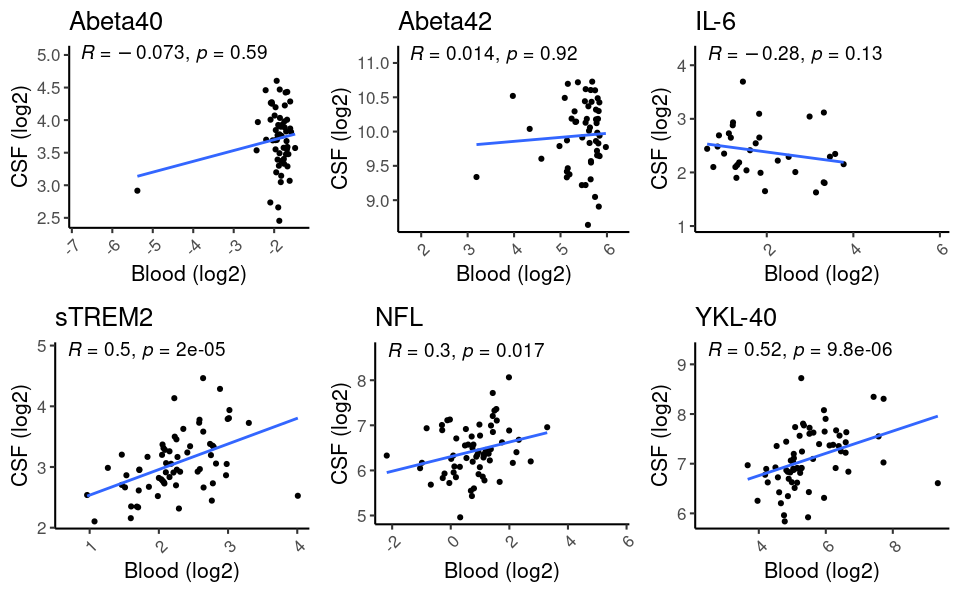
Concordance between CSF and blood NTK biomarkers was evident for sTREM2 (rho=.5, p<.001), NFL (rho=.3, p=.0.017) and YKL-40 (rho=.52, p<.001; [figure 1]).
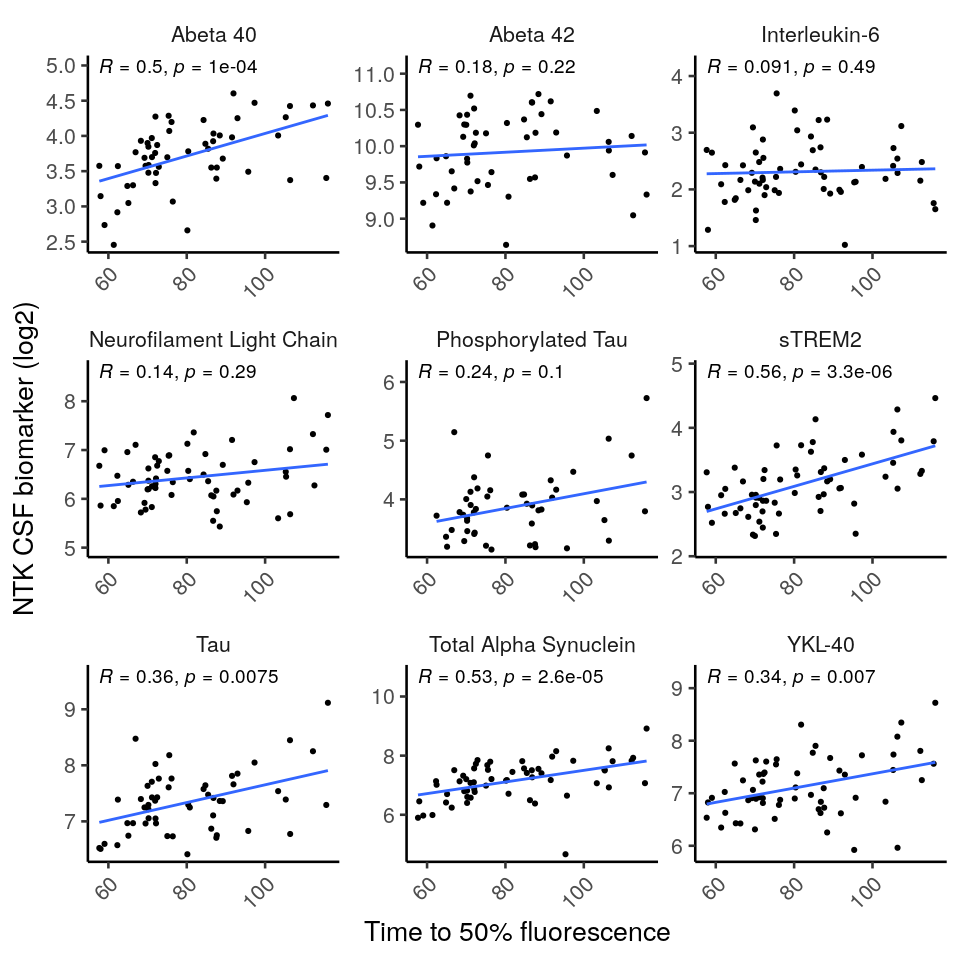
SAA kinetic parameters correlated with several CSF, but not blood (exception: sTREM2, p=0.026), NTK biomarker levels [figure 2].
Conclusion: Baseline biomarkers confirm high SAA positivity in a PASADENA subgroup, in line with similar cohorts, e.g. PPMI ([1], [2). The kinetics of the SAA correlated with a variety of CSF NTK biomarkers, which themselves were internally correlated, indicating that inter-individual differences in abundance of proteins might drive biomarker levels [3].
References: [1] Siderowf, Andrew, Luis Concha-Marambio, David-Erick Lafontant, Carly M. Farris, Yihua Ma, Paula A. Urenia, Hieu Nguyen, et al. “Assessment of Heterogeneity and Disease Onset in the Parkinson’s Progression Markers Initiative (PPMI) Cohort Using the α-Synuclein Seed Amplification Assay: A Cross-Sectional Study.” medRxiv, March 1, 2023. https://doi.org/10.1101/2023.02.27.23286156.
[2] Yoo, Dallah, Ji-In Bang, Choonghyun Ahn, Victoria Nyawira Nyaga, Young-Eun Kim, Min Ju Kang, and Tae-Beom Ahn. “Diagnostic Value of α-Synuclein Seeding Amplification Assays in α-Synucleinopathies: A Systematic Review and Meta-Analysis.” Parkinsonism & Related Disorders 104 (November 1, 2022): 99–109. https://doi.org/10.1016/j.parkreldis.2022.10.007.
[3] Schilde, Lukas M., Steffen Kösters, Simone Steinbach, Karin Schork, Martin Eisenacher, Sara Galozzi, Michael Turewicz, et al. “Protein Variability in Cerebrospinal Fluid and Its Possible Implications for Neurological Protein Biomarker Research.” PLOS ONE 13, no. 11 (November 29, 2018): e0206478. https://doi.org/10.1371/journal.pone.0206478.
To cite this abstract in AMA style:
T. Kustermann, G. Pagano, H. Svoboda, T. Nikolcheva, K. Taylor. Baseline fluid biomarkers in a phase 2 clinical trial in Parkinson’s Disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/baseline-fluid-biomarkers-in-a-phase-2-clinical-trial-in-parkinsons-disease/. Accessed January 8, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/baseline-fluid-biomarkers-in-a-phase-2-clinical-trial-in-parkinsons-disease/