Session Information
Date: Tuesday, September 24, 2019
Session Title: Parkinsonisms and Parkinson-Plus
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To complete the baseline analysis of clinical data from the PROSPECT-M-UK study and to assess the impact of applying current MDS PSP diagnostic criteria on the diagnostic rate of PSP compared to previous NINDS diagnostic criteria.
Background: Studying the natural history of atypical parkinsonian syndromes (APS), including PSP, CBS and MSA, along with genetic and serial biomarker measurements, are vital to: 1) the development of disease-specific markers; 2) discovering the biological determinants of disease progression.
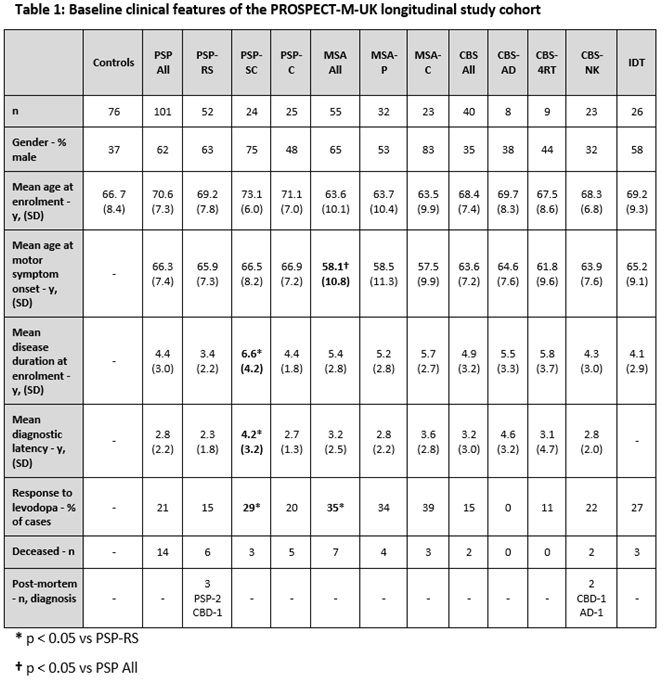
Method: PSP, CBS and MSA cases that fulfilled current diagnostic criteria were recruited from August 2015. We also recruited controls and indeterminate (IDT) cases that had features of APS but did not fulfil diagnostic criteria. PSP cases were initially recruited using NINDS criteria [1] and then reclassified using MDS diagnostic criteria [2] which allowed us to create the following groups: PSP-Richardson syndrome (PSP-RS); PSP-subcortical (PSP-SC), consisting of PSP-parkinsonism and pure akinesia with gait freezing cases; PSP-cortical (PSP-C), consisting of PSP-frontal and PSP/CBS overlap cases. MSA subjects were subdivided into MSA-parkinsonism (MSA-P) and MSA-cerebellar (MSA-C) cases. CBS subjects were subdivided into cases with CSF evidence (Tau:A-beta 1-42 ratio >1) of underlying AD pathology (CBS-AD), normal CSF Tau:A-beta 1-42 ratio (CBS-4RT) and cases without CSF analysis (CBS-NK). Group comparisons of baseline clinical data were carried out using t-testing.
Results: In total, we recruited 222 cases and 76 controls. By applying MDS diagnostic criteria, we increased our baseline number of PSP cases from 58 to 101. The baseline clinical features of the reclassified cohort are summarised below [table1]. In comparison to PSP, we found that MSA had a younger age at motor symptom onset and a better self-reported initial levodopa response. In addition, in comparison with PSP-RS, PSP-SC had a longer diagnostic latency and disease duration at recruitment.
Conclusion: The PROSPECT-M-UK study has recruited a large cohort of PSP, CBS and MSA patients. Application of MDS diagnostic criteria has increased the number of PSP cases that may be included in current/future clinical trials. PROSPECT-M-UK subjects are currently undergoing longitudinal collection of clinical, genetic and biomarker measures which will contribute to the discovery of disease-specific biomarkers and biological determinants of disease progression.
References: [1] Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996 Jul;47(1):1–9. [2] Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017 Jun;32(6):853–64.
To cite this abstract in AMA style:
E. Jabbari, J. Woodside, V. Chelban, A. Costantini, N. Holland, N. Leigh, D. Burn, N. Pavese, A. Gerhard, C. Kobylecki, M. Hu, A. Church, J. Rohrer, H. Houlden, J. Rowe, H. Morris. Baseline analysis of PROSPECT-M-UK: a longitudinal study of PSP, CBS and MSA [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/baseline-analysis-of-prospect-m-uk-a-longitudinal-study-of-psp-cbs-and-msa/. Accessed December 29, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/baseline-analysis-of-prospect-m-uk-a-longitudinal-study-of-psp-cbs-and-msa/