Session Information
Date: Tuesday, June 6, 2017
Session Title: Genetics (Non-PD)
Session Time: 1:45pm-3:15pm
Location: Exhibit Hall C
Objective: To characterize the phenotype and biochemical abnormalities associated with the R467X mutation in the SLCA20A2 gene.
Background: Mutations in the SLC20A2 gene are the most common cause of primary familial brain calcifications (PFBC). The R467X mutation in SLC20A2 has been described associated with paroxysmal kinesigenic dyskinesia (PKD) in a Japanese male. Ataxia is an unusual manifestation of PBFC. SLC20A2 encodes type III sodium-dependent phosphate transporter 2 (PIT2). A SLC20A2 knockout mouse displays progressive brain calcifications and elevated levels CSF phosphate (CSF-Pi). Very little is known about the natural history of SLC20A2-associated PFBC and its pathogenesis.
Methods: Clinical assessment with standard cognitive batteries, motor scales, imaging and biochemical analysis of plasma and CSF. For progression assessment we used the total calcification score (TCS) and a co-registration method.
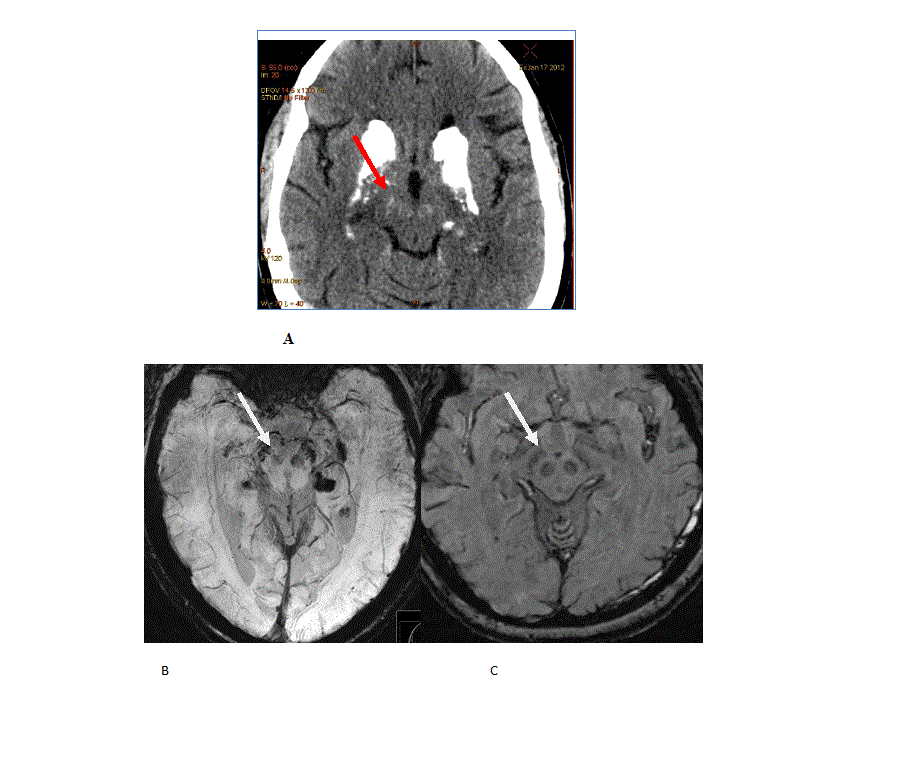
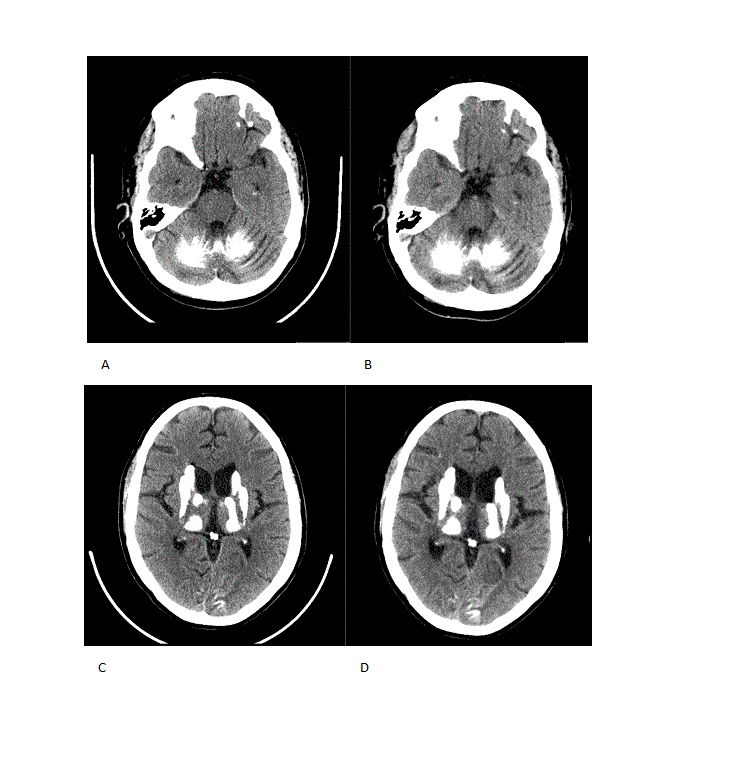
Results: A 54 year-old female with a past medical history of type 1 diabetes with poor glycemic control and hypertension was investigated for cognitive decline, personality change, impaired balance. And brain calcifications. Age at onset of cognitive symptoms was 42. Her father was affected by tremor but was not available to exam. Secondary causes of brain calcifications were ruled out first. The phenotype in this case consists of ataxia, supranuclear palsy, foot dystonia and subcortical dementia. Her SARA score at age 51 was 5.5 and two years later 7.5. Imaging revealed widespread brain calcifications in frontal and occipital cortex, basal ganglia, thalamus, cerebellum and midbrain including the substantia nigra (SN) (fig. 1 and 2). Her TCS was 55 and remained unchanged 3 years later. Using coregistration however we found progression of brain calcifications in the cerebellum and thalamus. In addition brain MRI demonstrated white matter hyperintensities outside of the calcified areas. In the CSF we found increased NfL and phosphate levels. There were no signs of damage to the blood-brain barrier. In March 2016 (age 55) a nephrotic syndrome was diagnosed, five months later the patient developed acute-onset left side hemiparesis due to an infarction in the contralateral internal capsule.
Conclusions: The severe phenotype we describe is very different to a previous report describing PKD associated with the R467X mutation in SLC20A2. The TCS here is among the highest reported so far (range 20-58 point in a previous publication). We found that coregistration is a more sensitive method to detect progression of brain calcifications. Elevated levels of NfL in the CSF is compatible with a the notion that PFBC are indeed neurodegenerative diseases. Elevated phosphate levels in this case as in the SLC20A2 knockout mouse suggests that this abnormality may be a potential biomarker for SLC20A2 mutations.
References: Yamada M, Tanaka M, Takagi M et al. Evaluation of SLC20A2 mutations that cause idiopathic basal ganglia calcification in Japan. Neurology, 2014 Feb 25;82(8):705–12. Wallingford MC, Chia J, Leaf EM et al. SLC20A2 deficiency in mice leads to elevated phosphate levels in cerebrospinal fluid and glymphatic pathway-associated arteriolar calcification, and recapitulates human idiopathic basal ganglia calcification. Brain Pathol, 2016 Jan 29.
To cite this abstract in AMA style:
M. Paucar, H. Almqvist, V. Jelic, G. Hagman, G. Jörneskog, S. Holmin, I. Björkhem, P. Svenningsson. Ataxia and increased cerebrospinal fluid phosphate associated with a mutation in the SLC20A2 gene [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/ataxia-and-increased-cerebrospinal-fluid-phosphate-associated-with-a-mutation-in-the-slc20a2-gene/. Accessed February 25, 2026.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/ataxia-and-increased-cerebrospinal-fluid-phosphate-associated-with-a-mutation-in-the-slc20a2-gene/