Session Information
Date: Thursday, June 8, 2017
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: To evaluate whether patient perceived improvement of Parkinson’s disease (PD) symptoms was associated with improvement in efficacy endpoints (mean daily OFF time and ON time without troublesome dyskinesia, and UPDRS-III motor scores) in a tozadenant clinical study.
Background: Tozadenant is an adenosine A2A antagonist under investigation as an adjunct to levodopa in patients with PD experiencing motor fluctuations. A phase 2b study of tozadenant 60 mg, 120 mg, 180 mg, 240 mg BID vs placebo was conducted in levodopa-treated patients with PD experiencing ≥2.5 h/day OFF time. The primary efficacy outcome, based on patient diaries, showed that compared with placebo, tozadenant 120 mg BID, 180 mg BID, and the combined doses (120 mg BID and 180 mg BID) significantly reduced mean daily OFF time by 1.1–1.2 h.1 Tozadenant 60 mg BID and 120 mg BID doses are currently being studied in phase 3 development.
Methods: A post-hoc analysis evaluated whether Patient Global Impression of Improvement (PGI-I) rating at week 12 was associated with changes in mean daily OFF time and ON time without troublesome dyskinesia, and changes in UPDRS-III score between baseline and week 12 in tozadenant 60 mg, 120 mg, and placebo groups. PGI-I rating was dichotomized into responders (any improvement at week 12) and nonresponders (no change or worsening). Only patients who completed the PGI-I rating at week 12 were included in the analysis.
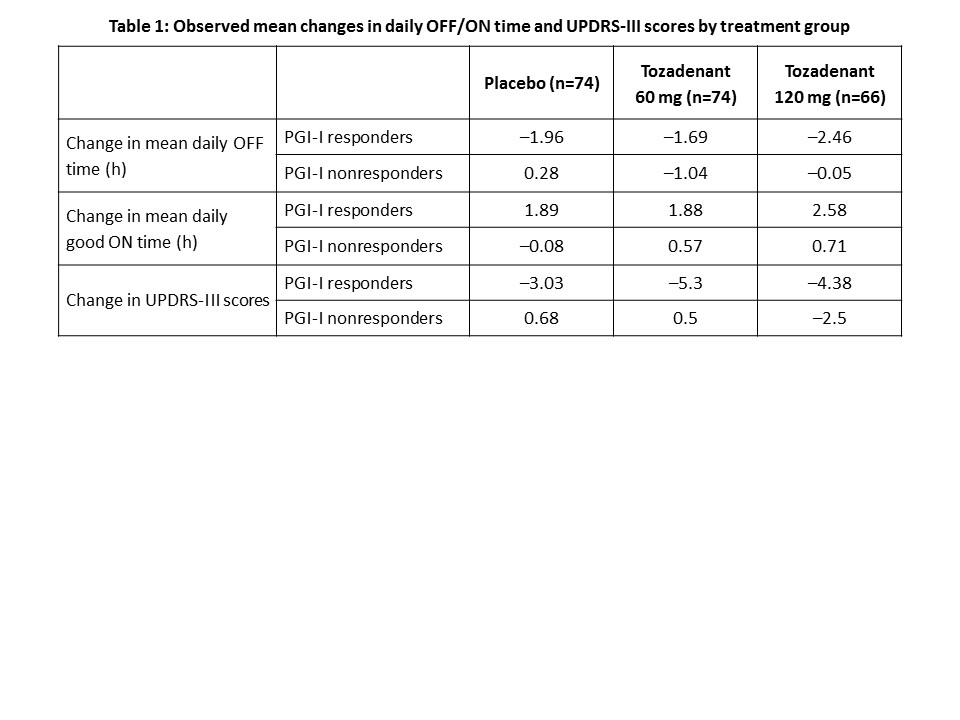
Results: Of the 247 subjects, 214 completed PGI-I rating at week 12 (placebo=74, 60 mg=74, 120 mg=66). Relative to placebo, a higher proportion of tozadenant-treated subjects reported a favorable PGI-I rating (placebo, 60 mg, 120 mg: 53%, 65%, 79%, respectively). Overall, PGI-I responders reported a reduction in mean daily OFF time vs nonresponders (–2.05 h vs –0.24 h; P<0.001), and an increase in mean daily good ON time without troublesome dyskinesia vs nonresponders (2.14 h vs 0.29 h; P<0.001). PGI-I responders also showed a reduction in UPDRS-III score compared with nonresponders (–4.31 vs 0.01; P<0.001). The observed mean changes by treatment group are shown in [table1].
Conclusions: In this exploratory analysis, PGI-I responders experienced a greater reduction in mean daily OFF time and increase in ON time without troublesome dyskinesia, and a greater improvement in observer-rated UPDRS-III scores compared with nonresponders.
References: 1.Hauser RA, Olanow CW, Kieburtz KD, et al. Tozadenant (SYN115) in patients with Parkinson’s disease who have motor fluctuations on levodopa: a phase 2b, double-blind, randomised trial. Lancet Neurol. 2014;13:767-776.
To cite this abstract in AMA style:
C. Kenney, A. Glass, W. Olanow. Associating patient impression of improvement with efficacy endpoints in Parkinson’s disease: A post-hoc analysis of a tozadenant study [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/associating-patient-impression-of-improvement-with-efficacy-endpoints-in-parkinsons-disease-a-post-hoc-analysis-of-a-tozadenant-study/. Accessed April 26, 2025.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/associating-patient-impression-of-improvement-with-efficacy-endpoints-in-parkinsons-disease-a-post-hoc-analysis-of-a-tozadenant-study/