Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate the local and systemic safety and tolerability of ABBV-951 (foscarbidopa/foslevodopa) delivered 24 h/day as a continuous subcutaneous infusion (CSCI) for up to 52 weeks in advanced Parkinson’s disease (aPD) patients.
Background: As PD progresses, oral drug regimens may become insufficient for symptom control and patients may transition to more advanced treatment options. Foscarbidopa/foslevodopa is a new soluble formulation of carbidopa and levodopa prodrugs being developed for the treatment of aPD as a CSCI via an external pump. In PD patients, foscarbidopa/foslevodopa was previously shown to be generally well-tolerated at individualized doses for up to 28 days.
Method: This Phase 3, open-label, single-arm study will examine the 52-week safety and tolerability of foscarbidopa/foslevodopa in aPD patients in an outpatient setting (NCT03781167). Key eligibility criteria include idiopathic, levodopa-responsive PD patients, with a minimum of 2.5 hours of “Off” time/day, whose motor fluctuations are inadequately controlled by conventional therapies. After a screening period, study visits will occur weekly during a dose optimization period and at weeks 6, 13, 26, 39, and 52 during the maintenance period. Adverse events (AEs), serious AEs and AEs of special interest will be monitored. Local tolerability will be assessed using the Infusion Site Evaluation Scale. Exploratory efficacy analyses will evaluate both motor and non-motor symptoms using PD diaries, symptom scales, and a wearable device.
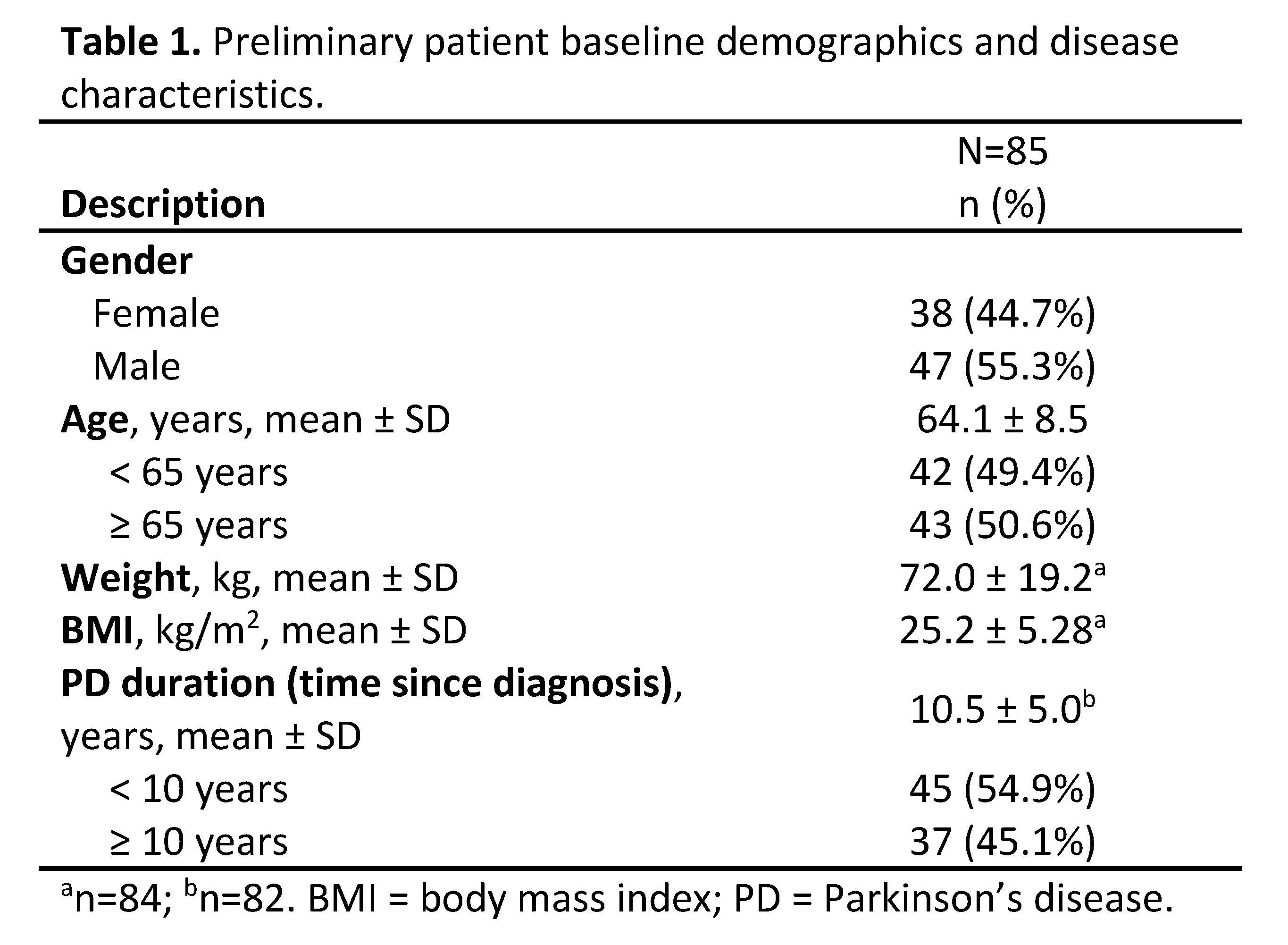
Results: The first patient was enrolled on 06 June 2019 and as of 24 January 2020, 125 patients have been enrolled from 55 study sites. As of 22 January 2020, the study population assessed (N=85) consisted of 55.3% male PD patients, with a mean age of 64.1 years, and an average PD duration of 10.5 years [Table 1].
Conclusion: Foscarbidopa/foslevodopa has the potential to provide a wide range of individualized doses of levodopa via a continuous 24 h/day infusion and offers a non-surgical treatment option for patients with aPD whose motor complications are inadequately controlled by conventional medications. The currently enrolled study population have baseline demographics and disease characteristics that are comparable to those of subjects enrolled in other studies with aPD patients.
To cite this abstract in AMA style:
M. Facheris, J. Streit, J. Jia, D. Standaert. Assessing the Safety and Tolerability of ABBV-951 (Foscarbidopa/Foslevodopa) in Advanced Parkinson’s Disease Patients During a 52-Week Phase 3 Study: Study Design and Updated Patient Baseline Characteristics [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/assessing-the-safety-and-tolerability-of-abbv-951-foscarbidopa-foslevodopa-in-advanced-parkinsons-disease-patients-during-a-52-week-phase-3-study-study-design-and-updated-patient-baseline/. Accessed December 20, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/assessing-the-safety-and-tolerability-of-abbv-951-foscarbidopa-foslevodopa-in-advanced-parkinsons-disease-patients-during-a-52-week-phase-3-study-study-design-and-updated-patient-baseline/