Category: Tremor
Objective: To assess differences in healthcare resource utilization (HCRU) and costs between patients with essential tremor (ET) receiving transcutaneous afferent patterned stimulation (TAPS) therapy and those receiving routine clinical care.
Background: TAPS therapy is an FDA-cleared, non-invasive wrist-worn neuromodulation device for the relief of action tremor (1,2). The therapy targets median and radial nerves with a frequency calibrated to each patient’s tremor. Previous clinical trials have demonstrated the efficacy and safety of TAPS therapy among patients with ET (1-4). Recent real-world data analyses (5,6) and preliminary data from a one-year pragmatic trial (7) further confirmed the benefit and safety of TAPS therapy in patients with ET. However, while the safety and effectiveness of TAPS are well-established, its potential impact on HCRU and costs remains unclear. Therefore, this study is designed to fill this gap by assessing the economic impact of TAPS use at home in patients with ET.
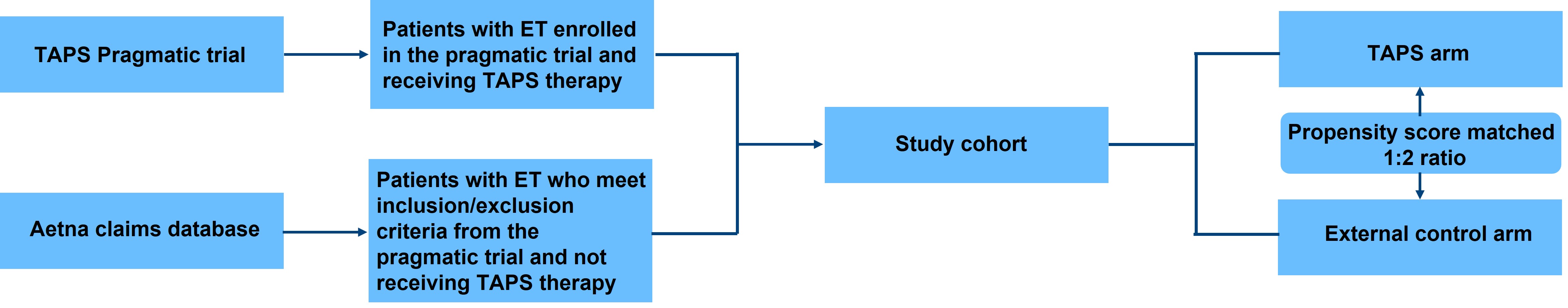
Method: Patients with ET who enrolled in a pragmatic TAPS device trial (ClinicalTrials.gov identifier: NCT05540626) and received TAPS therapy will be matched in a 1:2 ratio to control subjects with ET receiving routine clinical care identified in a large U.S. healthcare system based on propensity score (covariates including age, gender, geographic region, household income, payer type, Charlson comorbidity index, and ET treatment). Using Aetna® claims databases, HCRU (including emergency visits, prescriptions, and hospital admissions) and all-cause healthcare costs will be evaluated using generalized linear models (8).
Results: A total of 276 patients with ET were included in the pragmatic trial between May 2021 and February 2023 (7). Data collection for the pragmatic trial has been completed, and ongoing data analyses are in progress. Claims data for a 1-year follow-up are expected to be available by July 2024. All data analyses will be completed by September 2024 adhering to a pre-specified statistical analysis plan.
Conclusion: This study introduces a novel approach by identifying an external control arm to serve as a comparator for evaluating the economic impact of TAPS for the treatment of hand tremor in adult patients with ET in a real-world setting.
Figure 1. Study Design
References: 1. Pahwa R, Dhall R, Ostrem J, Gwinn R, Lyons K, Ro S, et al. An acute randomized controlled trial of noninvasive peripheral nerve stimulation in essential tremor. Neuromodulation. 2019;22(5):537–545. doi: 10.1111/ner.12930.
2. Isaacson SH, Peckham E, Tse W, Waln O, Way C, Petrossian MT, et al. Prospective Home-use Study on Non-invasive Neuromodulation Therapy for Essential Tremor. Tremor Hyperkinetic Mov. 2020;10:29. doi: 10.5334/tohm.59.
3. Yu JY, Rajagopal A, Syrkin-Nikolau J, Shin S, Rosenbluth KH, Khosla D, et al. Transcutaneous Afferent Patterned Stimulation Therapy Reduces Hand Tremor for One Hour in Essential Tremor Patients. Front Neurosci. 2020;14:530300. doi: 10.3389/fnins.2020.530300.
4. Lin PT, Ross EK, Chidester P, Rosenbluth KH, Hamner SR, Wong SH, et al. Noninvasive neuromodulation in essential tremor demonstrates relief in a sham-controlled pilot trial: Neuromodulation for Essential Tremor. Mov Disord. 2018;33(7):1182–1183. doi: 10.1002/mds.27350.
5. Brillman S, Colletta K, Borucki S, Lin PT, Waln O, Petrossian M, et al. Real-World Evidence of Transcutaneous Afferent Patterned Stimulation for Essential Tremor. Tremor Hyperkinetic Mov. 2022;12(1):27. doi: 10.5334/tohm.715.
6. Lu C, Khosla D, Kent A, Bronte-Stewart HM, Rosenbluth KH. Transcutaneous Afferent Patterned Stimulation for Essential Tremor: Real-World Evidence with Long Term Follow-Up. Tremor Other Hyperkinet Mov (N Y). 2023;13(1):29. doi: 10.5334/tohm.775.
7. Dai D, Fernandes J, Kim H, Coetzer H. Comparative Effectiveness of Transcutaneous Afferent Patterned Stimulation Therapy for Essential Tremor: A Randomized Pragmatic Clinical Trial. Tremor and Other Hyperkinetic Movement. 2023;13(1):38, pp.1-16. doi: 10.5334/tohm.798.
8. Dai D, Samiian A, Fernandes J, Coetzer H. Multiple comorbidities, psychiatric disorders, healthcare resource utilization and costs among adults with essential tremor: a retrospective observational study in a large US commercially insured and Medicare Advantage population. J Health Econ Outcomes Res. 2022;9(2):37–46. doi: 10.36469/001c.37307.
To cite this abstract in AMA style:
D. Dai, J. Fernandes, L. Samuel, C. Lu, S. Reitmaier, A. Berk. Assessing the Economic Impact of Transcutaneous Afferent Patterned Stimulation Therapy for Essential Tremor: A Real-World Evidence Study Protocol [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/assessing-the-economic-impact-of-transcutaneous-afferent-patterned-stimulation-therapy-for-essential-tremor-a-real-world-evidence-study-protocol/. Accessed February 21, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/assessing-the-economic-impact-of-transcutaneous-afferent-patterned-stimulation-therapy-for-essential-tremor-a-real-world-evidence-study-protocol/