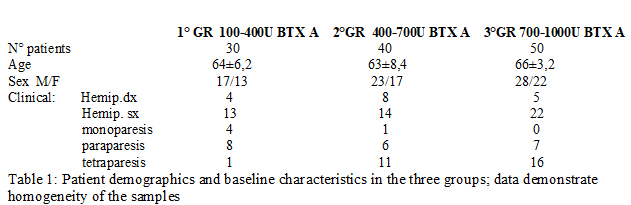Session Information
Date: Sunday, October 7, 2018
Session Title: Spasticity
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: The aim of this study is to object the efficiency and safety of 100 to 1000 units botulinum toxin treatment with modulation of spasticity according to the individual patient’s needs.
Background: One of the major treatments for tone modulation is botulinum toxin type A (BTX-A).To perform and optimize the rehabilitative treatment, an accurate clinical and instrumental evaluation of spasticity is needed to determine how this symptom is invalidating and to choose the best doses, muscles and times of inoculation in each patient.
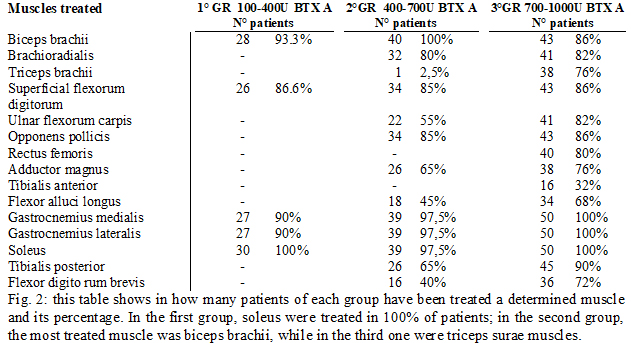
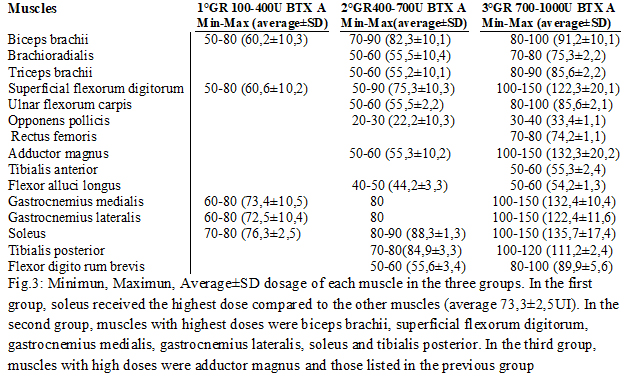
Methods: Patients were initially divided into three groups (fig.1) according to the botulinum toxin A dosage used at the beginning of study (IncobotulinumtoxinA, Xeomin®, Merz Pharma, 100 U/mL in saline): The doses were chosen depending on the severity of spasticity clinically evaluated and on the number of muscles treated (fig.2-3). The evaluation method applied, included Ashworth scale, FIM motor scales and myometric measurement (MyotonPRO®, tool that determines an objective value of muscle tone, elasticity. During the study, patients received rehabilitation, daily for the first 30 days after injection, followed by 3 days a week until next injection and stiffness). Statistical analysis: Wilkoxon matched-pairs signed ranks test, Wilkoxon rank-sum test (Mann-Whitney U test) were used.
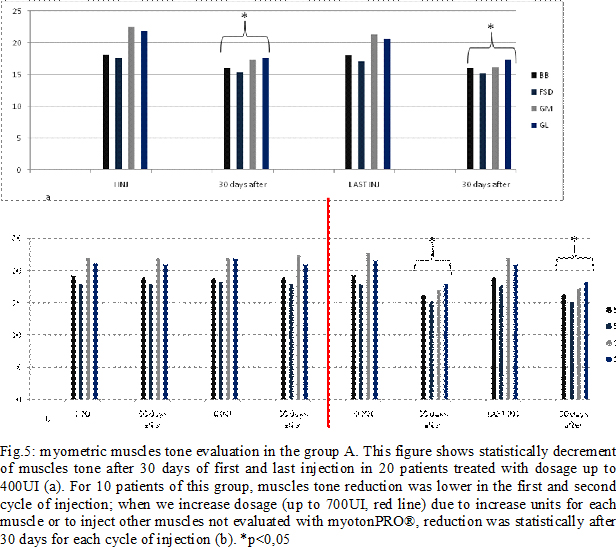
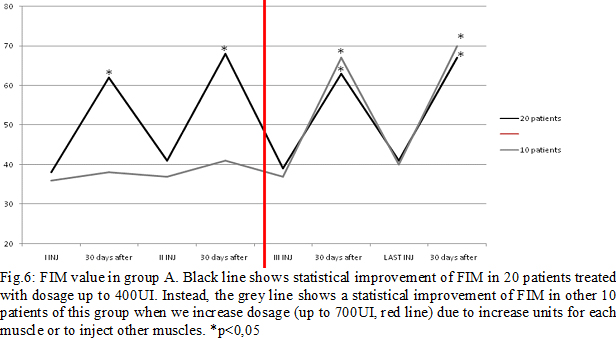
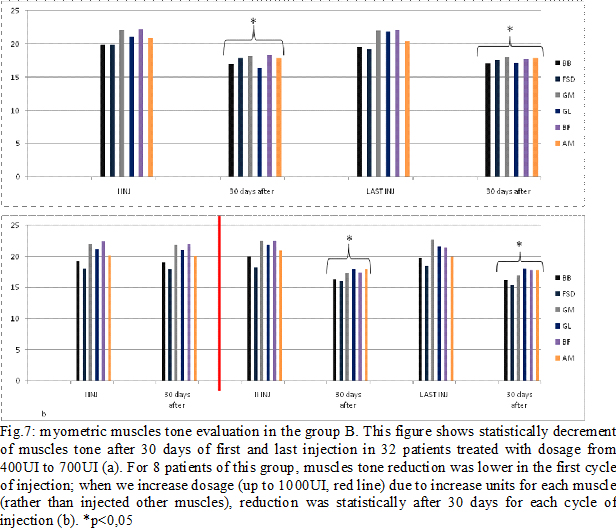
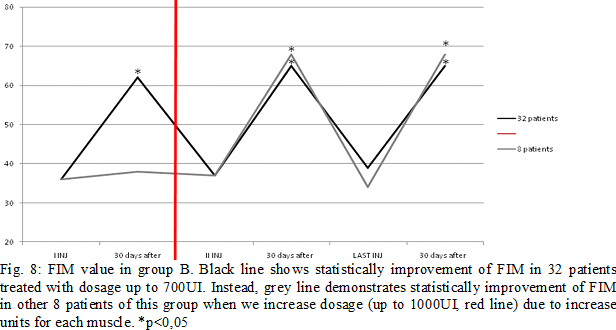
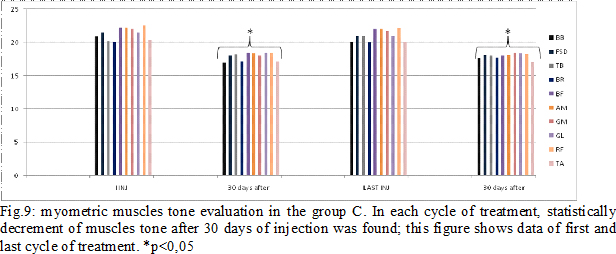
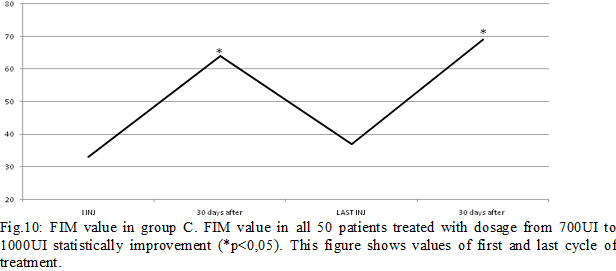
Results: Some patients switched to another group because the dosage used increased (Fig.4). In group A, at the third infiltration, we increased dosage in 10 patients (33%) due to poor clinical effects or to treat more muscles, so they switched in group B. In the following injections, these patients improved their clinical and instrumental measurement of spasticity (fig. 5-6). In group B, after the first infiltration, 8 patients (20%) showed no clinical and instrumental improvement, so we increased dosage (we treated muscles with greater dosage rather than treated more muscles) and they switched in group C. In the following injections, these 8 patients improved their clinical and instrumental measurement of spasticity (fig. 7-8).In the group C, average doses at the end of study was statistically significant compared to the beginning (fig. 9-10).
Conclusions: Considering larger Therapeutic Index and Therapeutic Window (from 100 to 1000 UI) of IncobotulinumtoxinA, we can better modulate spasticity. Therefore, we could introduce “appropriate treatment” and no “standard or high dosage treatment” concept; for the clinical features of each patient.
References: Riccardo Marvulli, Lucia Mastromauro, Ersilia Romanelli, Angela Lopopolo, Maria Dargenio, Fara Fornarelli, Elio Conte, , Pietro Fiore, Marisa Megna and Giancarlo Ianieri – How Botulinum toxin Type A- Occupational Therapy (OT)-Functional Electrical Stimulation (FES) Modify Spasticity and Functional Recovery in patients with Upper Limb Spasticity Post Stroke — Journal: Clinical Immunology, Endocrine & Metabolic Drugs Vol 3 Issue 1 (2015). G. Ianieri, R. Marvulli, E. Romanelli, L. Mastromauro, G.B. Marzo, A Dantone, R. D’argento, M.G. Ricchiuti – “Therapeutic switch from Onabotulinum toxin A (Botox) to Incobotulinum toxin A (Xeomin) in post stroke spasticity” – Jourrnal Rehabilitation Medicine Suppl – June 2015.
To cite this abstract in AMA style:
G. Ianieri, R. Marvulli. “Appropriate Doses Treatment” of IncobotulinumtoxinA in spasticity [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/appropriate-doses-treatment-of-incobotulinumtoxina-in-spasticity/. Accessed April 20, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/appropriate-doses-treatment-of-incobotulinumtoxina-in-spasticity/