Objective: The objetive of this study was to compare the benefit of APO and STN-DBS in aPD in terms of motor (off daily hours, UPDRS III, UPDRS IV and dyskinesia score), non-motor (NMSS, MADRS, SAS, PDSS-2), cognitive (MDRS and verbal fluency), quality of life (PDQ-39) and medication (levodopa and LEDD) in the same patient.
Background: Apomorphine infusion (APO) and subthalamic deep brain stimulation (STN-DBS) have shown their effectiveness in the treatment of advanced Parkinson’s disease (aPD). However, while several prospective, randomised, and multicenter clinical trials support STN-DBS, only one does the same for APO [1,2]. Data have shown that patients with APO are usually older, have more prolonged disease and a more severe phenotype [1-9]. Only two non-randomised and prospective studies have compared APO and STN-DBS in patients meeting the intervention [10,11].
Method: We prospectively analysed 20 aPD patients (Table 1) in three situations, baseline (ON-MED), APO and STN-DBS, for a time from 12 to 18 months. APO and STN-DBS phase were stable for 6 months. APO and STN-DBS evaluations were separated by 6 months to avoid disease progression.
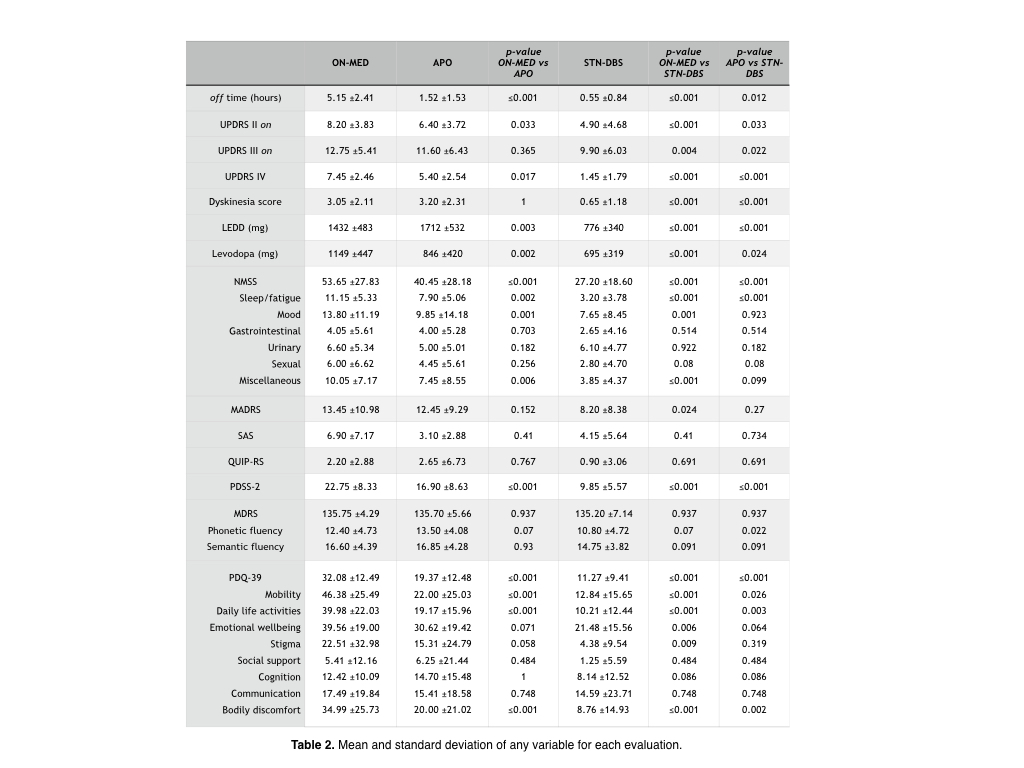
Results: Compared to baseline, APO and STN-DBS reduced off-time by 70.5% and 89.3% (between both treatments, p =0.012), UPDRS IV by 27.5% and 80.5% (p ≤0.001), levodopa by 26.8% and 39.5% (p =0.024), NMSS by 24.6% and 49.3% (p ≤0.001), MADRS by 7.4% and 39.0 (p =0.27), SAS by 51.1% and 39.9% (p =0.734), PDSS-2 by 25.7% and 56.7% (P ≤0.001) and PDQ-39 by 39.6% and 64.9% (p ≤0.001). Dyskinesia improved dramatically after STN-DBS (78.7%) and did not change with APO. Global cognition did not modify with any therapy, but phonetic fluency worsened after STN-DBS (p =0.022). Results and statistical analysis are shown in table 2.
Conclusion: This is the first prospective study that compares APO and STN-DBS in the same patient. APO and STN-DBS have improved motor and non-motor symptoms and quality of life of these aPD patients compared to baseline. STN-DBS has been the most effective treatment, but APO has shown a notable benefit for this patient profile’s motor symptoms. Overall cognition did not change with APO or STN-DBS, except for a worsening of phonetic fluency after the intervention. We should not delay an appropriate treatment for aPD.
References: Katzenschlager R, Poewe W, Rascol O, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, doubleblind, randomised, placebo-controlled trial. Lancet Neurol. 2018;17(9):749-759. Katzenschlager R, Poewe W, Rascol O, et al. Long-term safety and efficacy of apomorphine infusion in Parkinson’s disease patients with persistent motor fluctuations: Results of the open-label phase of the TOLEDO study. Parkinsonism Relat Disord. 2021;83:79-85. Martínez-Martín P, Reddy P, Antonini A, Henriksen T, Katzenschlager R, Odin P, et al. Chronic subcutaneous infusion therapy with apomorphine in advanced Parkinson’s disease compared to conventional therapy: a real life study of non motor effect. J Parkinsons Dis. 2011;1(2):197-203. Drapier S, Gillioz AS, Leray E, Péron J, Rouaud T, Marchand A, et al. Apomorphine infusion in advanced Parkinson’s patients with subthalamic stimulation contraindications. Parkinsonism Relat Disord. 2012;18(1):40-44. Martínez-Martín P, Reddy P, Katzenschlager R, Antonini A, Todorova A, Odin P, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord. 2015;30(4):510-516. Sesar A, Fernández-Pajarín G, Ares B, Rivas MT, Castro A. Continuous subcutaneous apomorphine infusion in advanced Parkinson’s disease: 10-year experience with 230 patients. J Neurol. 2017;264(5):946-954. Borgemeester RW, Drent M, van Laar T. Motor and non-motor outcomes of continuous apomorphine infusion in 125 Parkinson’s disease patients. Parkinsonism Relat Disord. 2016;23:17-22. Houvenaghel JF, Drapier S, Duprez J, Robert GH, Riou A, Drapier D, et al. Effects of continuous subcutaneous apomorphine infusion in Parkinson’s disease without cognitive impairment on motor, cognitive, psychiatric symptoms and quality of life. J Neurol Sci. 2018;395:113-118. Dafsari HS, Martinez-Martin P, Rizos A, Trost M, Dos Santos Ghilardi MG, Reddy P, et al. EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord. 2019;34(3):353-365. De Gaspari D, Siri C, Landi A, Cilia R, Bonetti A, Natuzzi F, et al. Clinical and neuropsychological follow up at 12 months in patients with complicated Parkinson’s disease treated with subcutaneous apomorphine infusion or deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatry. 2006;77(4):450-453. Alegret M, Valldeoriola F, Martí M, Pilleri M, Junqué C, Rumià J, et al. Comparative cognitive effects of bilateral subthalamic stimulation and subcutaneous continuous infusion of apomorphine in Parkinson’s disease. Mov Disord. 2004;19(12):1463-1469.
To cite this abstract in AMA style:
G. Fernández-Pajarín, A. Sesar, B. Ares, I. Jimenez-Martín, M. Gelabert-González, E. Arán-Echabe, JL. Relova, A. Castro. Apomorphine infusion before subthalamic deep brain stimulation. A prospective comparative study in the same 20 patients. [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/apomorphine-infusion-before-subthalamic-deep-brain-stimulation-a-prospective-comparative-study-in-the-same-20-patients/. Accessed April 27, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/apomorphine-infusion-before-subthalamic-deep-brain-stimulation-a-prospective-comparative-study-in-the-same-20-patients/