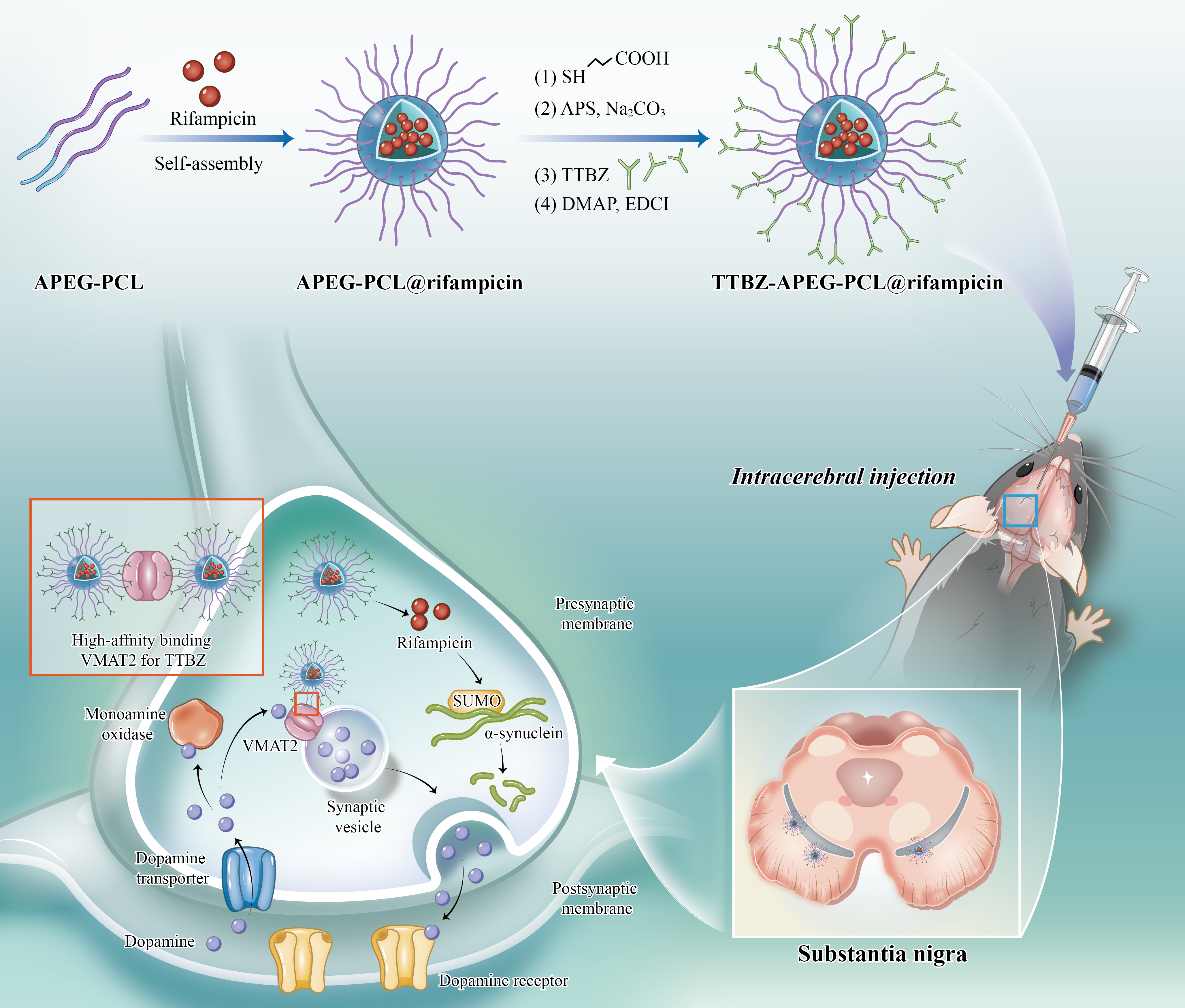
Objective: There were accumulating evidence proving that rifampicin could protect neurons from apoptosis in Parkinson’s disease in-vitro model by regulating SUMO modification of α-synuclein. However, we had determined that the concentration of rifampicin in animals’ midbrain tissue was only 10 % of that in cell culture medium, so that the effect of rifampicin in vivo was not as stable as that in vitro. To deal with this problem, we tried to packaged rifampicin with TTBZ labeled nanoparticles as a nanomedicine for the therapy of Parkinson’s disease.
Background: Evidence suggests that alpha-synuclein (α-syn) aggregation in the midbrain has a vital function in Parkinson’s disease (PD) pathogenesis; therefore, clearance of α-syn represents an urgent unmet clinical need. Rifampicin has great potential in treating PD byinhibiting the aggregation of α-syn in vitro. however, it remains a challenge to achieve dopaminergic neurons-targeted and an effective local concentration of rifampicin in vivo.
Method: Herein, a TTBZ (3-(2-hydroxy-3-isobutyl-10-methoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a] isoquinolin- 9-yloxy) propyl 4-methylbenzenesulfonate) decorated polymersome TTBZ-allyl poly (ethylene glycol)-poly (ε-caprolactone) (TTBZ-APEG-PCL, T-NPs) was developed for the targeted delivery of rifampicin . More importantly, a micro-osmotic pump system was applied for the intracerebroventricular delivery of R-T-NPs, which provided continuous neuroprotection against α-syn aggregation in the midbrain of an α-syn-induced PD mice model.
Results: In this study, we injected the nanomedicine into the lateral ventricle of PD mice model. It resulted in a significant improvement in the concentration of rifampicin in the mice’s substantia nigra tissue. Meanwhile, the pathology change in substantia nigra and motor dysfunction of PD mice were significantly reversed. What’s more, the inhibition of cellular SUMO modification level as well as its catalytic enzyme PIAS1 were observed in substantia nigra neurons of PD mice model.
Conclusion: Our studies implied PIAS1 might be related with sumoylation modification of α-synuclein, which contributed to the misfolding and aggregation of α-synuclein. We hypothesized that the nanomedicine with rifampicin could inhibit the sumoylation modification of α-synuclein and prevent the neuron from apoptosis by regulating the expression of PIAS1 in dopaminergic neuron.
References: 1. Manzanza NO, Sedlackova L, Kalaria RN. α-synuclein post-translational modifications: implications for pathogenesis of lewy body disorders. Front Aging Neurosci. 2021 Jun
25;13:690293.
2. Stojkovska I, Wani WY, Zunke F, Belur NR, Pavlenko EA, Mwenda N, Sharma K, Francelle L, Mazzulli JR. Rescue of α-synuclein aggregation in Parkinson’s patient neurons by
synergistic enhancement of ER proteostasis and protein trafficking. Neuron. 2022 Feb2;110(3):436-451.e11.
3. Xu J, Wei C, Xu C, Bennett MC, Zhang G, Li F, Tao E. Rifampicin protects PC12 cells against MPP+-induced apoptosis and inhibits the expression of an α-synuclein multimer. Brain Res. 2007 Mar 30;1139:220-5.
4. Liang Z, Chan HYE, Lee MM, Chan MK. A SUMO1-derived peptide targeting SUMO-interacting motif inhibits α-synuclein aggregation. Cell Chem Biol. 2021 Feb
18;28(2):180-190.e6.
5. Umeda T, Hatanaka Y, Sakai A, Tomiyama T. Nasal Rifampicin improves cognition in a mouse model of dementia with lewy bodies by reducing α-synuclein oligomers. Int J Mol Sci. 2021 Aug 6;22(16):8453.
To cite this abstract in AMA style:
D. Lin, K. Huang. APEG-PCL Nanoparticles Containing Rifampicin Target Dopaminergic Neurons to reduce α-synuclein aggregation [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/apeg-pcl-nanoparticles-containing-rifampicin-target-dopaminergic-neurons-to-reduce-%ce%b1-synuclein-aggregation/. Accessed December 31, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/apeg-pcl-nanoparticles-containing-rifampicin-target-dopaminergic-neurons-to-reduce-%ce%b1-synuclein-aggregation/