Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: Objective: Formulations of Mucuna pruriens (MP) seed products are readily available through the internet and health food stores, and are used by some Parkinson’s Disease (PD) patients as an alternative to conventional levodopa/carbidopa medication. The purpose of this study was to examine the L-Dopa content of a range of popular MP products, in order to assess the veracity of label claims.
Background: Background: MP is a pantropical legume that possesses a unique and significant polypharmacy of constituents. The seeds contain 3-10% levodopa (L-Dopa), MP has documented use in traditional Ayurvedic medicine since 1500 BCE to treat diseases resembling PD. Pilot studies in PD show that MP seed powder has similar effects to conventional levodopa/carbidopa medication. Studies suggest that MP seed preparations led to a considerably faster onset of effect, reflected in shorter latencies to peak L-Dopa plasma concentrations. The rapid onset of action and longer on time without concomitant increase in dyskinesia on MP suggest that natural source of L-Dopa might possess advantages over conventional L-Dopa treatment.
Method: Methods: Six different brands of MP product were ordered through the internet. Certificates of analysis were obtained where possible. A standard amount of each product was extracted using methanol:formic acid, and analyzed using reversed-phase high performance liquid chromatography (HPLC) with ultraviolet and fluorescence detection. L-Dopa content was calculated using a standard curve prepared using L-Dopa as reference.
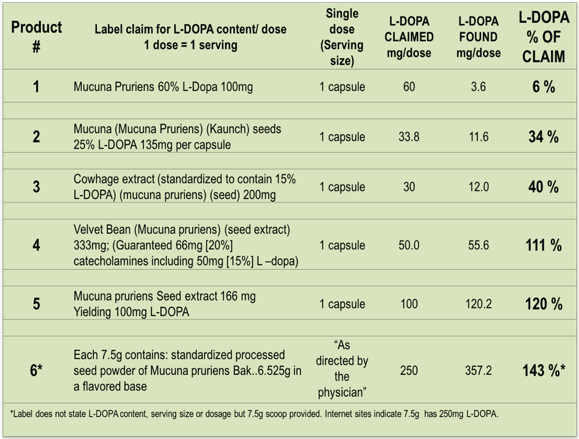
Results: Results: A wide discrepancy of L-Dopa ranging from 6% -143% of label claim was observed in the products examined. The claimed L-Dopa content ranged from 25 to 250 mg per dose for the six products. HPLC analysis revealed that only two of the products had L-DOPA values close to the value claimed. The remaining products contained considerably less L-Dopa, <10% in two cases, than implied on the label. Certificates of analysis suggested that not all manufacturers routinely measure L-Dopa content of their MP product (table 1).
Conclusion: Conclusion: Four of six products examined showed a large discrepancy between label claim and L-Dopa content, independently measured by HPLC. This finding warrants further investigation as these discrepancies could impact both patients, and the outcome of clinical studies using these products.
References: References: (1) Katzenschlager R, Evans A, Manson A, et al. Mucuna pruriens in parkinson’s disease: A double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry 1997; 75: 1672-1677. (2) Manyam BV, An alternative medicine treatment for Parkinson’s disease: results of a multicenter clinical trial. HP-200 in Parkinson’s Disease Study Group. J Altern Complement Med 1995; 1(3): 249-255. (3) Siddhuraju P, Becker K. Rapid reversed-phase high performance liquid chromatographic method for the quantification of Levodopa (L-3, 4-dihydroxyphenylalanine), non-methylated and methylated tetrahydroisoquinoline compounds from mucuna beans. Food Chem 2001; 72: 389-394.
To cite this abstract in AMA style:
T. Denne. Analysis of Levodopa Content in Commercial Formulations of Mucuna pruriens Seeds Used in Integrative Treatment of Parkinson’s Disease [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/analysis-of-levodopa-content-in-commercial-formulations-of-mucuna-pruriens-seeds-used-in-integrative-treatment-of-parkinsons-disease/. Accessed June 30, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/analysis-of-levodopa-content-in-commercial-formulations-of-mucuna-pruriens-seeds-used-in-integrative-treatment-of-parkinsons-disease/