Category: Parkinson’s Disease: Clinical Trials
Objective: Prove safety and feasibility of delivering allogeneic bone marrow-derived mesenchymal stem cells (MSC) intravenously in escalated doses to patients with idiopathic Parkinson’s disease (PD).
Background: Neuroinflammation plays a crucial role in the pathogenesis of PD, supporting the rationale for using MSC as immunomodulatory therapy for restoring homeostasis to the neuronal-glial microenvironment.
Method: 12-month single-center open-label dose-escalation phase 1 study. 20 subjects with mild/moderate PD were sequentially assigned to one of four doses: 1, 3, 6, or 10 X 106MSCs/kg given as a single intravenous infusion; Groups A,B,C,D respectively and evaluated at 3, 12, 24, and 52 weeks post-infusion. Primary outcome safety measures were absence of immediate transfusion reaction, study-related adverse events, organ damage or immunogenic reactions. Secondary outcomes were therapy’s impact on peripheral markers, PD progression, and changes in brain perfusion.
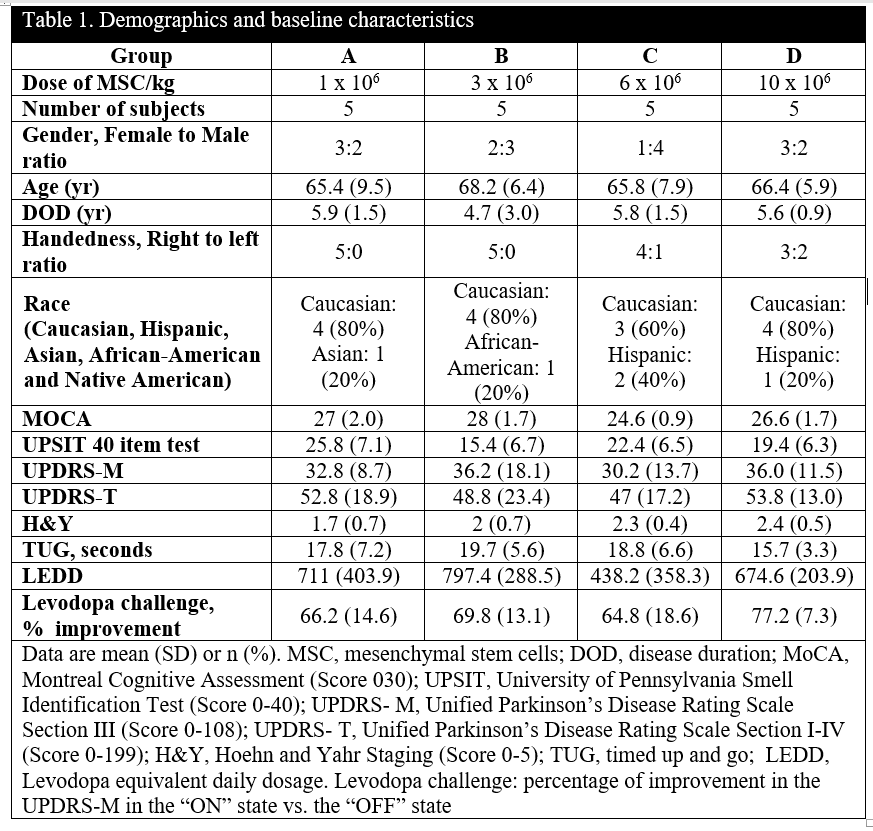
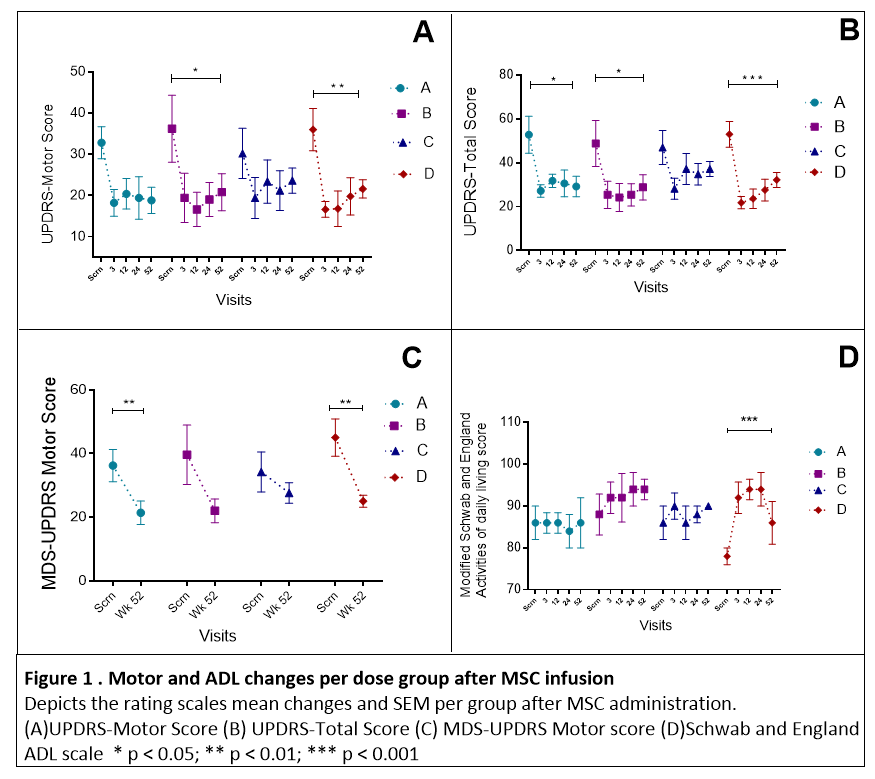
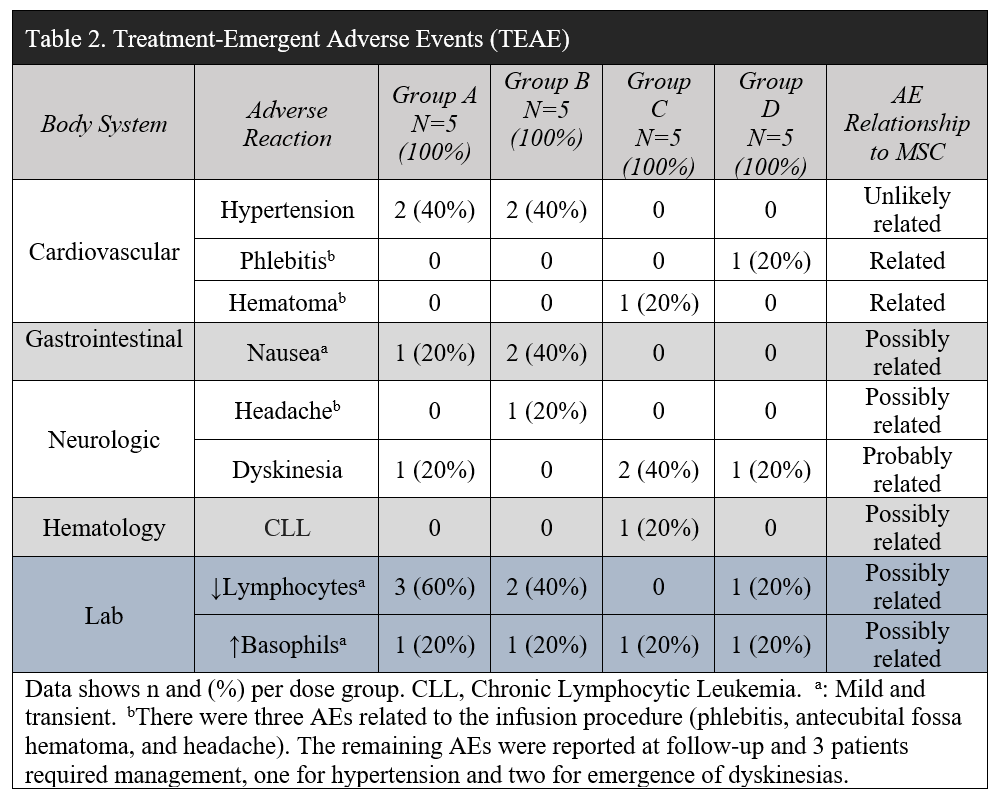
Results: Baseline characteristics appeared similar across groups except for cognition (p<0.05) [Table 1]. Twenty subjects received a single IV infusion; during the first 24 hours post-infusion, three patients reported transient treatment-emergent adverse events (TEAEs), one phlebitis, one antecubital fossa hematoma, and one headache. Most common TEAEs were dyskinesias (25%, N=5) and hypertension (20%, N=4). One study SAE happened in a patient with a 4-year history of lymphocytosis who developed asymptomatic chronic lymphocytic leukemia [Table 2]. There was no response to donor HLA over the 12-month study. At 52 weeks CCL2 declined 15% (p<0.01), CCL22 29% (p<0.05), along with an increase in BDNF 46% (p<0.05). The highest dose had the most significant effect on reducing OFF UPDRS motor -14.4 (p<0.01) and total scores (-20.8, p<0.001) [Figure 1]. Main effects of time and region (p<0.001) on brain perfusion occurred with a significant increase in the subthalamic nucleus (p=0.042) at 24 weeks.
Conclusion: This study demonstrates that a single infusion of allogeneic MSC is safe and well-tolerated in mild/moderate PD patients. Our data points towards a treatment effect that relies on peripheral immune modulation. There is a potential signal for clinical improvement, but this should be interpreted with caution based on both the limited sample size and the purpose of the study.
To cite this abstract in AMA style:
M. Schiess, J. Suescun, M. Doursout, C. Adams, C. Green, J. Saltarrelli, S. Savitz, T. Ellmore. Allogeneic bone marrow-derived mesenchymal stem cells safety and tolerability in idiopathic Parkinson’s disease [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/allogeneic-bone-marrow-derived-mesenchymal-stem-cells-safety-and-tolerability-in-idiopathic-parkinsons-disease/. Accessed December 27, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/allogeneic-bone-marrow-derived-mesenchymal-stem-cells-safety-and-tolerability-in-idiopathic-parkinsons-disease/