Category: Technology
Objective: Examine actigraphy wear compliance in 5 neurological-disorders clinical trials using an ActiGraph GT9X Link with additional compliance features
Background: Wrist worn biometric devices are powerful tools for at-home assessment of neurological patient physiology and well-being, but more evidence on typical device wear compliance is needed [1]. This evidence can facilitate 1) informed decisions on wearables deployment in clinical research, 2) accurate sample size calculations when wearables are used, and 3) compliance improvement methods in clinical trials. Combining active tasks with wrist worn devices for passive measurement is becoming more common in neurological studies [2], but the value of this approach cannot be realized unless high patient compliance is achieved.
Method: Wear compliance data from 5 neurological trials using at-home digital measures collected from a GT9X Link were extracted from the Koneksa data repository. Wear compliance was determined per patient per day based on study requirements. Means (per patient and per study) were computed as 100*[# compliant days]/[# expected days]. Median-patient compliance was computed by finding the median of all patients’ mean compliance. Logistic regression with mixed effects was used to assess differences across time.
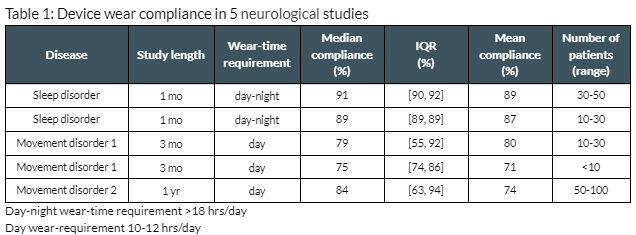
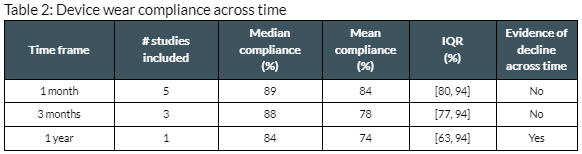
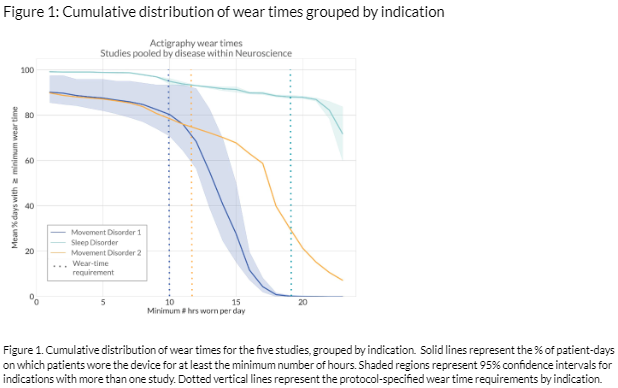
Results: The median actigraphy device wear compliance in neurological patients was 89% across 5 studies in 3 indications and 143 patients [table 1]. The wear time requirements ranged from 10-19 hours/day [figure 1]. In the first month, median compliance was 89% across all studies. Three studies, with daytime-only wear requirements, lasted ≥3 months. Across the first 3 months, the median compliance was 88% with no evidence of decline. In a year-long study with a daytime-only wear requirement, the median compliance was 84%. Significant compliance decline was observed across the year [table 2].
Conclusion: Actigraphy device wear compliance in neurological studies is not yet well defined. Studies typically aim for >80% compliance [3] and may base sample and effect size calculations on this target. Presented data suggest this goal is achievable in the context of well-run studies. As expected, wear compliance tends to decrease with time, but even at the one-year mark, median compliance remained >80%. Compliance by day on study may be an appropriate threshold measure for inclusion of patient data into a study’s analysis dataset.
References: [1] Olaye I, et al. “Recommendations for Defining and Reporting Adherence Measured by Biometric Monitoring Technologies: Systematic Review”. J Med Internet Res 2022;24(4):e33537. DOI: 10.2196/33537
[2] Lipsmeier, F, et al. “Reliability and Validity of the Roche PD Mobile Application for Remote Monitoring of Early Parkinson’s Disease. Nature Scientific Reports. 2022;12:12081. DOI: 10.1038/s41598-022-15874-4
[3] Baumgartner PC et al. “A Systematic Review of Medication Adherence Thresholds Dependent of Clinical Outcomes”. Front Pharmacol. 2018;9:1290. DOI:10.3389/fphar.2018.01290
To cite this abstract in AMA style:
J. Lavine, A. Pearlmutter, K. Momeni, R. Ellis. Actigraphy wear compliance across CNS disorders [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/actigraphy-wear-compliance-across-cns-disorders/. Accessed January 8, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/actigraphy-wear-compliance-across-cns-disorders/