Objective: To investigate neutralizing antibody (NAb) formation in three large Phase 3 studies with incobotulinumtoxinA, a botulinum neurotoxin type A (BoNT-A) with no complexing proteins, in children/adolescents with cerebral palsy (CP) who received multipattern spasticity treatment.
Background: NAbs have been linked to secondary non-response to BoNT-A injection; this concerning and still controversial issue is relevant when treating conditions like pediatric spasticity.
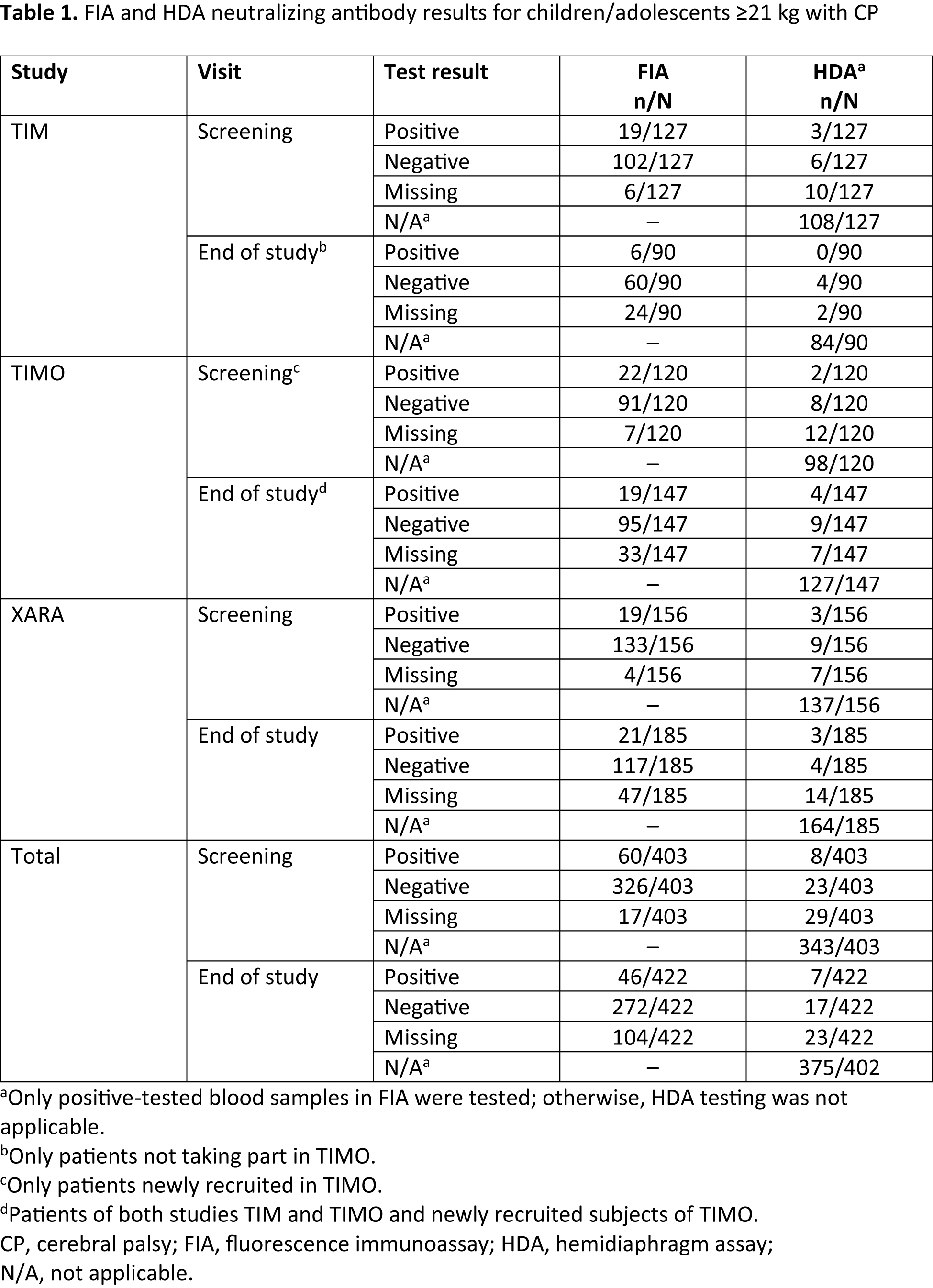
Method: Pediatric patients with lower-limb (LL), upper-limb (UL), or combined LL/UL spasticity were enrolled (2–17 years of age with uni-/bilateral CP and Ashworth Scale score ≥2 in clinical patterns for treatment). Patients received total body incobotulinumtoxinA doses of ≤16–20 U/kg (max. 400–500 U) depending on the study (TIM: NCT01893411; TIMO: NCT01905683; XARA: NCT02002884) and Gross Motor Function Classification System level I–V, for up to six injection cycles (ICs). The occurrence of NAbs against BoNT-A was investigated in those ≥21 kg at screening and end of study. Blood samples were first analyzed using a fluorescence immunoassay (FIA) for antibodies, and then positive samples were tested for NAbs using a hemidiaphragm assay.
Results: Overall, 907 patients received treatment (mean [SD] age 6.7 [4.2] years; 59.6% male; body weight 23.3 [13.9] kg). In total, 386/403 (95.8%) and 318/422 (75.4%) patients with body weight ≥21 kg were tested using FIA at screening and end of study, respectively (table1), with 150/403 (37.2%) and 167/422 (39.6%) being toxin-naïve. Eleven individual patients tested positive for NAbs: four at screening only, four at end of study only, and three at both screening and end of study. All of these patients had previously been treated with other BoNT-As: five with onabotulinumtoxinA only, one with abobotulinumtoxinA only, and five with both onabotulinumtoxinA/abobotulinumtoxinA. None of these patients developed a secondary non-response to incobotulinumtoxinA. No toxin-naïve patients developed NAbs after treatment with incobotulinumtoxinA.
Conclusion: No NAb formation was observed after up to six incobotulinumtoxinA ICs (dose up to 500 U) in toxin-naïve children/adolescents with CP.
Presented at TOXINS 2021 (Jan 16–17).
To cite this abstract in AMA style:
H. Chambers, P. Kaňovský, A. Schroeder, E. Dabrowski, T. Geister, A. Hanschmann, I. Pulte, M. Althaus, M. Banach, D. Gaebler-Spira, F. Heinen. Absence of neutralizing antibody formation during incobotulinumtoxinA treatment of spasticity in botulinum toxin-naïve children with cerebral palsy: pooled analysis of Phase 3 studies [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/absence-of-neutralizing-antibody-formation-during-incobotulinumtoxina-treatment-of-spasticity-in-botulinum-toxin-naive-children-with-cerebral-palsy-pooled-analysis-of-phase-3-studies/. Accessed January 4, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/absence-of-neutralizing-antibody-formation-during-incobotulinumtoxina-treatment-of-spasticity-in-botulinum-toxin-naive-children-with-cerebral-palsy-pooled-analysis-of-phase-3-studies/