Category: Parkinson's Disease: Neuroimaging
Objective: We sought to explore the concordance between the dynamics of different rs-fMRI regional indices in order to better understand intrinsic brain activity (IBA) alterations and pathophysiological mechanisms in Parkinson’s disease(PD).
Background: Researches using resting-state functional magnetic resonance imaging (rs-fMRI) have applied different regional measurements to IBA of patients with PD. Most previous studies have only examined the static characteristics of IBA in patients with PD, neglecting the dynamic features. The degree of integration is represented by the concordance between the different dynamic regional indices. The study investigated the volume-wise and voxel-wise concordance between dynamics of these regional measures. Whether the concordance between regional indicators is abnormal in PD patients remains a direction to be explored.
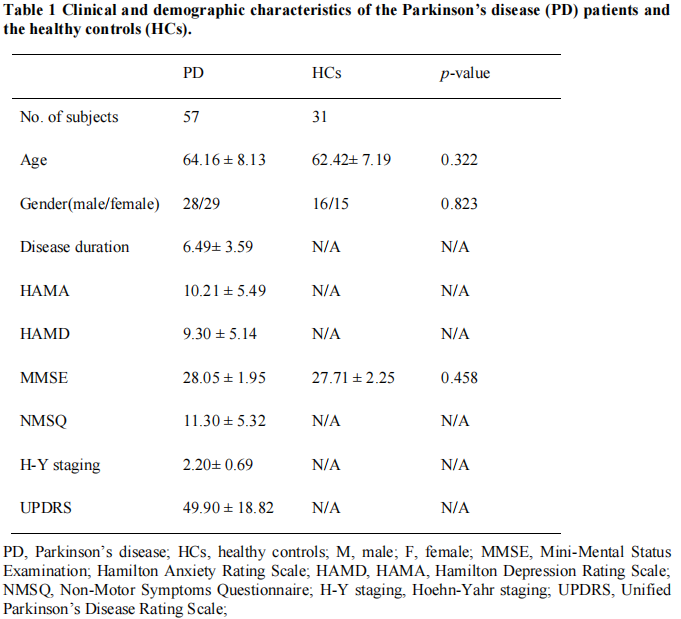
Method: This study included 31 healthy controls (HCs) and 57 PD patientsto calculate the volume-wise (across voxels) and voxel-wise (across periods) concordance using a sliding time window approach. This allowed us to compare the concordance of dynamic alterations in frequently used metrics such as degree centrality (DC), global signal connectivity (GSC), voxel-mirrored heterotopic connectivity (VMHC), the amplitude of low-frequency fluctuations (ALFF), and regional homogeneity (ReHo). We analyzed the changes of concordance indices in the PD patients andinvestigated the relationship between aberrant concordance values and clinical/neuropsychological assessments in the PD patients.
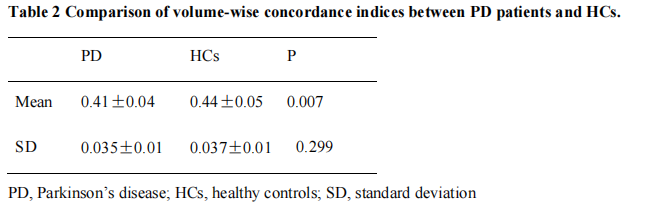
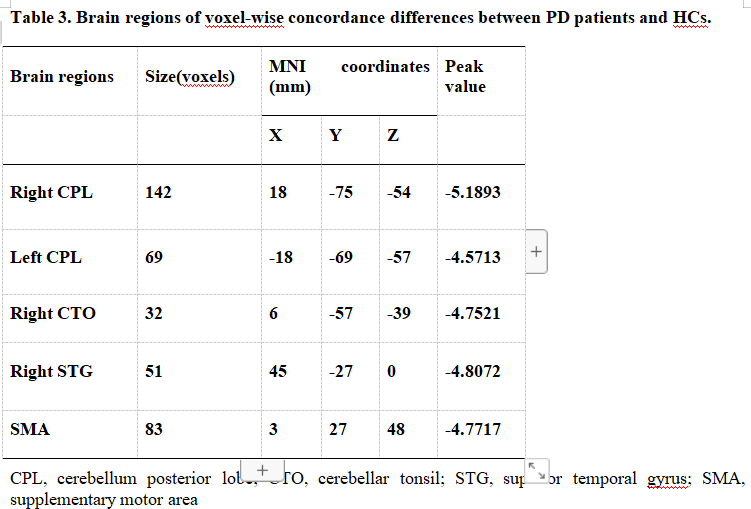
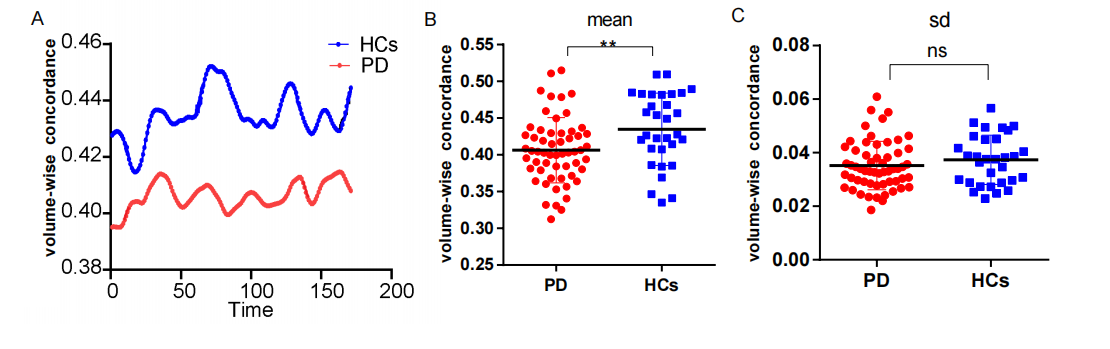
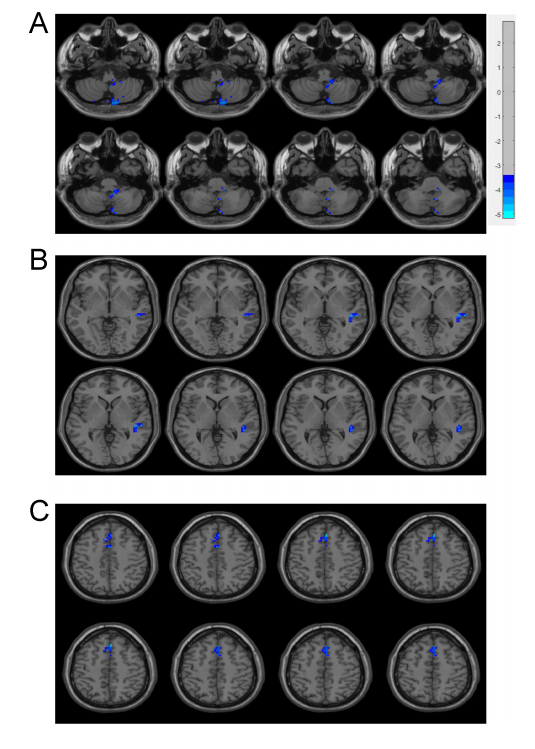
Results: We found that, compared with the HCs, the PD patients had lower volume concordance in the whole brain and lower voxel-wise concordance in the posterior cerebellar lobe,
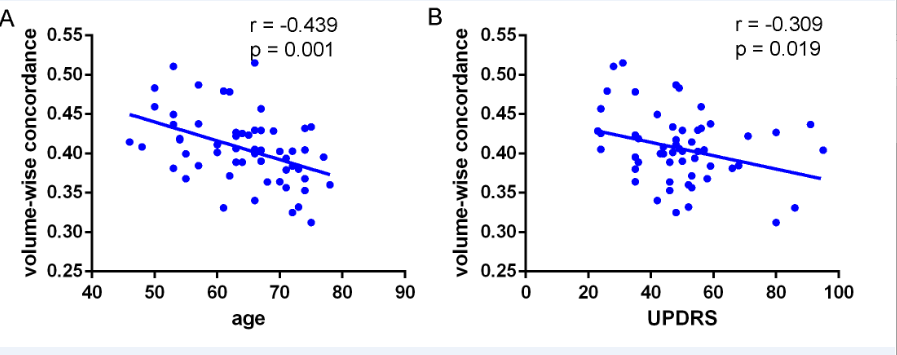
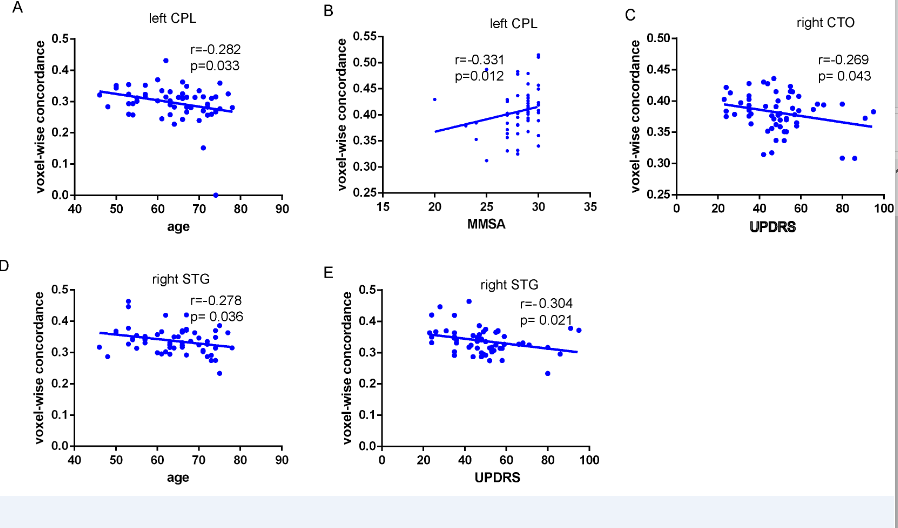
cerebellar tonsils, superior temporal gyrus, and supplementary motor region. We also found negative correlations between these concordance alterations and patients’ age. The exploratory results contribute to a better understanding of IBA alterations and pathophysiological mechanisms in PD.
Conclusion: In conclusion, this study demonstrates that PD patients have altered patterns of coherence obtained from rs-fMRI across several routine IBA measurements. We believe that concordance measures can provide novel insights into IBA mechanisms in PD .
References: Reference
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. doi:10.1016/j.neuroimage.2007.07.007.
Chen, X., Lu, B., and Yan, C. G. (2018). Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum. Brain Mapp. 39, 300–318. doi:10.1002/hbm.23843.
Chen, X., Onur, O. A., Richter, N., Fassbender, R., Gramespacher, H., Befahr, Q., et al. (2021)Concordance of Intrinsic Brain Connectivity Measures Is Disrupted in Alzheimer’s Disease.Brain Connect. 00, 1–12. doi:10.1089/brain.2020.0918.
de Vos, F., Koini, M., Schouten, T. M., Seiler, S., van der Grond, J., Lechner, A., et al. (2018). A comprehensive analysis of resting state fMRI measures to classify individual patients with Alzheimer’s disease. Neuroimage 167, 62–72. doi:10.1016/j.neuroimage.2017.11.025.
Filippi, M., Spinelli, E. G., Cividini, C., and Agosta, F. (2019). Resting State Dynamic Functional Connectivity in Neurodegenerative Conditions : A Review of Magnetic Resonance Imaging Findings. 13, 1–8. doi:10.3389/fnins.2019.00657. 322
Fiorenzato, E., Strafella, A. P., Kim, J., Schifano, R., Weis, L., Antonini, A., et al. (2019). Dynamic functional connectivity changes associated with dementia in Parkinson’s disease. Brain 142, 2860–2872. doi:10.1093/brain/awz192.
Fu, Z., Tu, Y., Di, X., Du, Y., Pearlson, G. D., Turner, J. A., et al. (2018). Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic functional connectivity: An application to schizophrenia. Neuroimage 180, 619–631. doi:10.1016/j.neuroimage.2017.09.035.
Guo, M. R., Ren, Y., Yu, H. M., Yang, H. G., Cao, C. H., Li, Y. M., et al. (2020). Alterations in Degree Centrality and Functional Connectivity in Parkinson’s Disease Patients With Freezing of Gait: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Neurosci. 14, 1– 12. doi:10.3389/fnins.2020.582079.
Hacker, C. D., Perlmutter, J. S., Criswell, S. R., Ances, B. M., and Snyder, A. Z. (2012). Resting state functional connectivity of the striatum in Parkinson’s disease. Brain 135, 3699–3711. doi:10.1093/brain/aws281
Harel, M., Stern, N., Attar, F., et al. (2014). Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect. 4, 395–403. doi:10.1089/brain.2014.0244.
Hanakawa, T., Goldfine, A. M., and Hallett, M. (2017). A common function of basal ganglia-cortical circuits subserving speed in both motor and cognitive domains. eNeuro 4.
doi:10.1523/ENEURO.0200-17.2017.
Helmich, R. C., Hallett, M., Deuschl, G., Toni, I., and Bloem, B. R. (2012). Cerebral causes and consequences of parkinsonian resting tremor: A tale of two circuits? Brain 135, 3206–3226. doi:10.1093/brain/aws023.
Hilker, R., Voges, J., Weisenbach, S., Kalbe, E., Burghaus, L., Ghaemi, M., et al. (2004). Subthalamic Nucleus Stimulation Restores Glucose Metabolism in Associative and Limbic Cortices and in Cerebellum: Evidence from a FDG-PET Study in Advanced Parkinson’s Disease. J. Cereb. Blood Flow Metab. 24, 7–16. doi:10.1097/01.WCB.0000092831.44769.09.
Hong Yu (2008). Role of hyeractive cerebellum and motor cortex in PD. Neuroimage 35, 222–233. doi:10.1016/j.neuroimage.2006.11.047
Hosenfeld, B., Bos, E. H., Wardenaar, K. J., Conradi, H. J., van der Maas, H. L. J., Visser, I., et al. (2015). Major depressive disorder as a nonlinear dynamic system: Bimodality in the frequency distribution of depressive symptoms over time. BMC Psychiatry 15, 1–9. doi:10.1186/s12888- 015-0596-5.
Hutchison, R. M., Womelsdorf, T., Allen, E. A., Bandettini, P. A., Calhoun, V. D., Corbetta, M., et al. (2013). Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 80, 360–378. doi:10.1016/j.neuroimage.2013.05.079. Jankovic, J. (2005). Progression of Parkinson Disease. Arch. Neurol. 62, 351.doi:10.1001/archneur.62.3.351.
Jia, X., Li, Y., Li, K., Liang, P., and Fu, X. (2019). Precuneus dysfunction in Parkinson’s disease with mild cognitive impairment. Front. Aging Neurosci. 11, 1–9. doi:10.3389/fnagi.2018.00427.
Kalia, L. V., and Lang, A. E. (2015). Parkinson’s disease. Lancet 386, 896–912. doi:10.1016/S0140- 6736(14)61393-3.
Kim, J., Criaud, M., Cho, S. S., Díez-Cirarda, M., Mihaescu, A., Coakeley, S., et al. (2017). Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain 140, 2955– 2967. doi:10.1093/brain/awx233.
Lewis, M. M., Slagle, C. G., Smith, A. B., Truong, Y., Bai, P., McKeown, M. J., et al. (2007). Task specific influences of Parkinson’s disease on the striato-thalamo-cortical and cerebello-thalamo- cortical motor circuitries. Neuroscience 147, 224–235. doi:10.1016/j.neuroscience.2007.04.006.
Li, K., Su, W., Li, S. H., Jin, Y., and Chen, H. B. (2018). Resting State fMRI: A Valuable Tool for Studying Cognitive Dysfunction in PD. Parkinsons. Dis. 2018. doi:10.1155/2018/6278649.
Liu, F., Wang, Y., Li, M., Wang, W., Li, R., Zhang, Z., et al. (2016). Dynamic Functional Network Connectivity in Idiopathic Generalized Epilepsy with Generalized Tonic – Clonic Seizure. 00, 1–17. doi:10.1002/hbm.23430.
Luo, C., Guo, X., Song, W., Zhao, B., Cao, B., Yang, J., et al. (2015). Decreased Resting-State Interhemispheric Functional Connectivity in Parkinson ’ s Disease. 2015.Nachev, P., Kennard, C., and Husain, M. (2008). Functional role of the supplementary and pre- supplementary motor areas. Nat. Rev. Neurosci. 9, 856–869. doi:10.1038/nrn2478.
Palmer, S. J., Li, J., Wang, Z. J., and McKeown, M. J. (2010). Joint amplitude and connectivity compensatory mechanisms in Parkinson’s disease. Neuroscience 166, 1110–1118. doi:10.1016/j.neuroscience.2010.01.012.
Pan, P., Zhan, H., Xia, M., Zhang, Y., Guan, D., and Xu, Y. (2016). Aberrant regional homogeneity in Parkinson ’ s disease : a voxel -wise meta-analysis of resting-state functional magnetic resonance imaging. Elsevier Ltd doi:10.1016/j.neubiorev.2016.11.018.
Pan, P., Zhang, Y., Liu, Y., Zhang, H., Guan, D., and Xu, Y. (2017). Abnormalities of regional brain function in Parkinson’s disease: A meta-analysis of resting state functional magnetic resonance imaging studies. Sci. Rep. 7, 1–10. doi:10.1038/srep40469.
Paus, T., Tomaiuolo, F., Otaky, N., Petrides, M., Atlas, J., Morris, R., et al. (1996). Human Cingulate
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., and Petersen, S. E. (2012). NeuroImage Spurious but systematic correlations in functional connectivity MRI networks arise from subjectmotion. Neuroimage 59, 2142–2154. doi:10.1016/j.neuroimage.2011.10.018.
Prodoehl, J., Spraker, M., Corcos, D., Comella, C., and Vaillancourt, D. (2010). Blood oxygenation level-dependent activation in basal ganglia nuclei relates to specific symptoms in de novo Parkinson’s disease. Mov. Disord. 25, 2035–2043. doi:10.1002/mds.23360.
Rana, A. Q., Ahmed, U. S., Chaudry, Z. M., and Vasan, S. (2015). Parkinson’s disease: A review of non-motor symptoms. Expert Rev. Neurother. 15, 549–562.
doi:10.1586/14737175.2015.1038244.
Raza, C., Anjum, R., and Shakeel, N. ul A. (2019). Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 226, 77–90. doi:10.1016/j.lfs.2019.03.057.
Reichmann, H., Brandt, M. D., and Klingelhoefer, L. (2016). The nonmotor features of Parkinson’s disease: Pathophysiology and management advances. Curr. Opin. Neurol. 29, 467–473.doi:10.1097/WCO.0000000000000348.
Sen, S., Kawaguchi, A., Truong, Y., Lewis, M. M., and Huang, X. (2010). Dynamic changes in cerebello-thalamo-cortical motor circuitry during progression of Parkinson’s disease.
Neuroscience 166, 712–719. doi:10.1016/j.neuroscience.2009.12.036. Tahmasian, M., Eickhoff, S. B., Giehl, K., Schwartz, F., Herz, D. M., Drzezga, A., et al. (2017).
Resting-state functional reorganization in Parkinson’s disease: An activation likelihood estimation meta-analysis. Cortex 92, 119–138. doi:10.1016/j.cortex.2017.03.016.
Tessitore, A., Cirillo, M., and De Micco, R. (2019). Functional Connectivity Signatures ofParkinson’s Disease. J. Parkinsons. Dis. 9, 637–652. doi:10.3233/JPD-191592.
Vikene, K., Skeie, G. O., and Specht, K. (2019). Compensatory task-specific hypersensitivity in bilateral planum temporale and right superior temporal gyrus during auditory rhythm an omission processing in Parkinson ’ s disease. Sci. Rep., 1–9. doi:10.1038/s41598-019-48791-0.
Wang, J., Zhang, J. R., Zang, Y. F., and Wu, T. (2018a). Consistent decreased activity in the putamen
in Parkinson’s disease: A meta-analysis and an independent validation of resting-state fMRI. Gigascience 7. doi:10.1093/gigascience/giy071.
Wang, J., Zhang, J., and Zang, Y. (2018b). Consistent decreased activity in the putamen in Parkinson ’ s disease : a meta-analysis and an independent validation of resting-state fMRI. 1– 13. doi:10.1093/gigascience/giy071.
Weingarten, C. P., Sundman, M. H., Hickey, P., and Chen, N. kuei (2015). Neuroimaging ofParkinson’s disease: Expanding views. Neurosci. Biobehav. Rev. 59, 16–52. doi:10.1016/j.neubiorev.2015.09.007.
Wu, T., and Hallett, M. (2013). The cerebellum in Parkinson’s disease. Brain 136, 696–709.doi:10.1093/brain/aws360.
Wu, T., Wang, L., Hallett, M., Li, K., and Chan, P. (2010). Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain 133, 2394–2409.
doi:10.1093/brain/awq151.
Yan, C. G., Yang, Z., Colcombe, S. J., Zuo, X. N., and Milham, M. P. (2017). Concordance among indices of intrinsic brain function: Insights from inter-individual variation and temporal dynamics. Science China Press doi:10.1016/j.scib.2017.09.015.
Yan, C., Wang, X., Zuo, X., and Zang, Y. (2016). DPABI : Data Processing & Analysis for ( Resting-State ) Brain Imaging. Neuroinformatics. doi:10.1007/s12021-016-9299-4.
Yang, H., Zhou, X. J., Zhang, M. ming, Zheng, X. ning, Zhao, Y. lei, and Wang, J. (2013). Changes in spontaneous brain activity in early Parkinson’s disease. Neurosci. Lett. 549, 24–28. doi:10.1016/j.neulet.2013.05.080.
Yang, Y., Zha, X., Zhang, X., Ke, J., Hu, S., Wang, X., et al. (2021). Dynamics and Concordance Abnormalities Among Indices of Intrinsic Brain Activity in Individuals With Subjective Cognitive Decline: A Temporal Dynamics Resting-State Functional Magnetic Resonance Imaging Analysis. Front. Aging Neurosci. 12, 1–12. doi:10.3389/fnagi.2020.584863.
Yu, H., Sternad, D., Corcos, D. M., and Vaillancourt, D. E. (2007). Role of hyperactive cerebellumand motor cortex in Parkinson’s disease. Neuroimage 35, 222–233.
doi:10.1016/j.neuroimage.2006.11.047.
Yu, Y., Li, Z., Lin, Y., Yu, J., Peng, G., Zhang, K., et al. (2019). Depression Affects Intrinsic Brain Activity in Patients With Mild Cognitive Impairment. 13, 1–9. doi:10.3389/fnins.2019.01333.
Yue, Y., Jiang, Y., Shen, T., Pu, J., Lai, H. Y., and Zhang, B. (2020). ALFF and ReHo Mapping Reveals Different Functional Patterns in Early- and Late-Onset Parkinson’s Disease. Front.Neurosci. 14, 1–9. doi:10.3389/fnins.2020.00141.
Zang, Y. F., Yong, H., Chao-Zhe, Z., Qing-Jiu, C., Man-Qiu, S., Meng, L., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91. doi:10.1016/j.braindev.2006.07.002.
Zang, Y., Jiang, T., Lu, Y., He, Y., and Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400. doi:10.1016/j.neuroimage.2003.12.030
Zhang, C., Dou, B., Wang, J., Xu, K., Zhang, H., Sami, M. U., et al. (2019). Dynamic Alterations of Spontaneous Neural Activity in Parkinson’s Disease: A Resting-State fMRI Study. Front. Neurol. 10. doi:10.3389/fneur.2019.01052.
Zhang, J., Wei, L., Hu, X., Xie, B., Zhang, Y., Wu, G. R., et al. (2015). Akinetic-rigid and tremor- dominant Parkinson’s disease patients show different patterns of intrinsic brain activity. Park.Relat. Disord. 21, 23–30. doi:10.1016/j.parkreldis.2014.10.017.
Zhu, J., Zhu, D. min, Qian, Y., Li, X., and Yu, Y. (2018). Altered spatial and temporal concordance among intrinsic brain activity measures in schizophrenia. J. Psychiatr. Res. 106, 91–98. doi:10.1016/j.jpsychires.2018.09.015.
Zou, Q. H., Zhu, C. Z., Yang, Y., Zuo, X. N., Long, X. Y., Cao, Q. J., et al. (2008). An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 172, 137–141. doi:10.1016/j.jneumeth.2008.04.012.
Zuo, X. N., Ehmke, R., Mennes, M., Imperati, D., Castellanos, F. X., Sporns, O., et al. (2012). Network centrality in the human functional connectome. Cereb. Cortex 22, 1862–1875.doi:10.1093/cercor/bhr269.
Zuo, X. N., Kelly, C., Di Martino, A., Mennes, M., Margulies, D. S., Bangaru, S., et al. (2010). Growing together and growing apart: Regional and sex differences in the lifespan
developmental trajectories of functional homotopy. J. Neurosci. 30, 15034–15043. doi:10.1523/JNEUROSCI.2612-10.2010.
To cite this abstract in AMA style:
T. Yuan, L. Kai, S. Wen. Aberrant Volume-wise and Voxel-wise Concordance among Dynamic Intrinsic Brain Activity Indices in Parkinson’s Disease [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/aberrant-volume-wise-and-voxel-wise-concordance-among-dynamic-intrinsic-brain-activity-indices-in-parkinsons-disease/. Accessed April 26, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/aberrant-volume-wise-and-voxel-wise-concordance-among-dynamic-intrinsic-brain-activity-indices-in-parkinsons-disease/