Session Information
Date: Monday, September 23, 2019
Session Title: Rare Genetic and Metabolic Diseases
Session Time: 1:45pm-3:15pm
Location: Les Muses Terrace, Level 3
Objective: Propose a new systems approach model for PKAN, investigate treatment, and establish biologic and genetic markers.
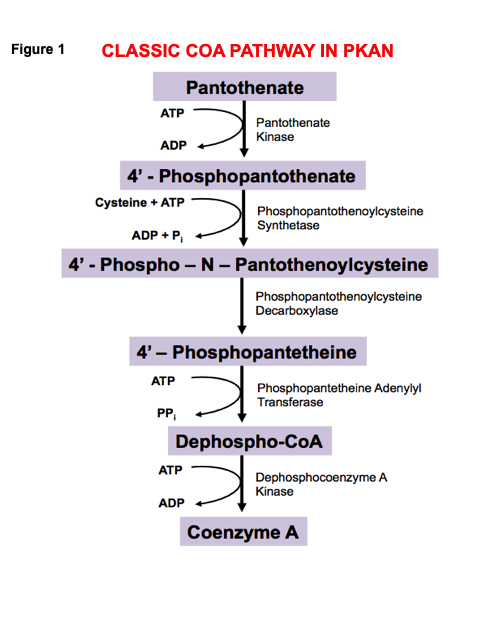
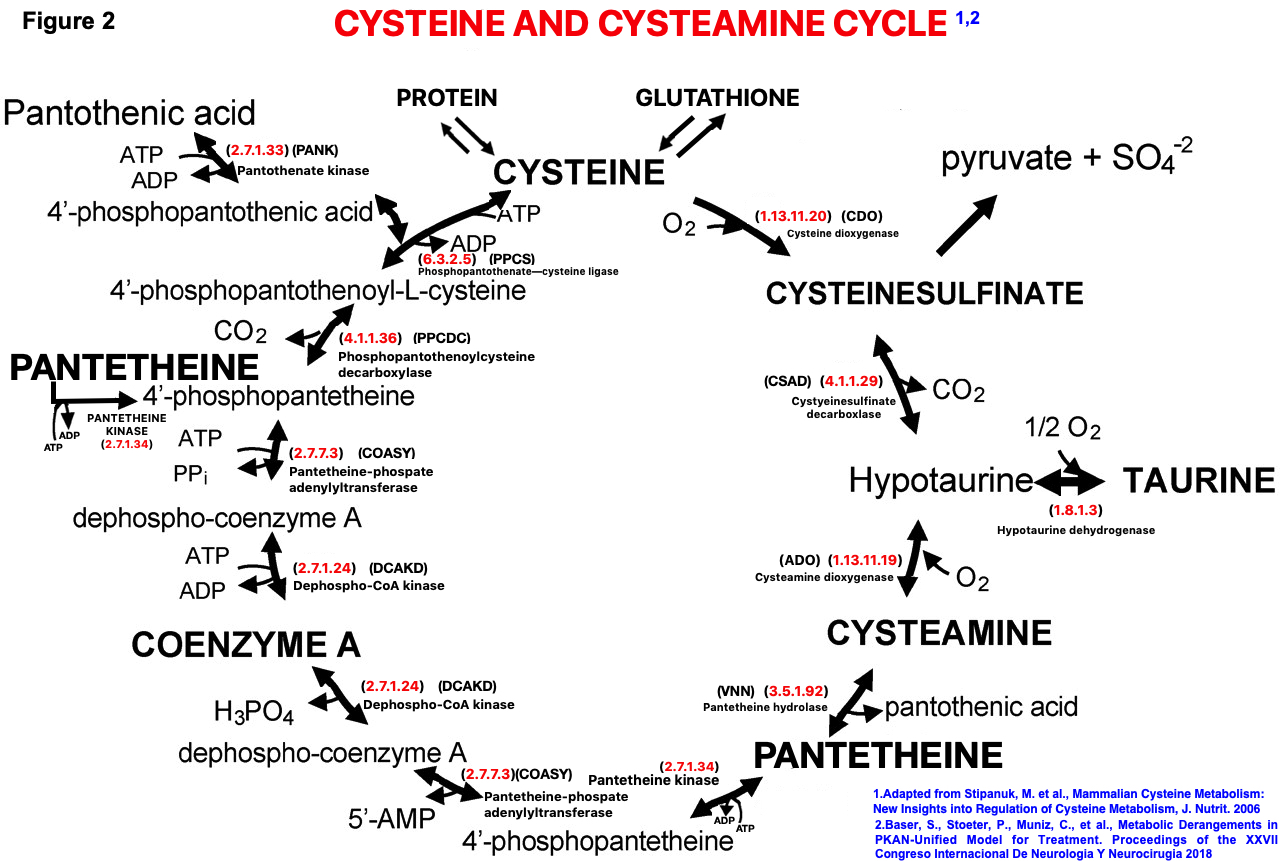
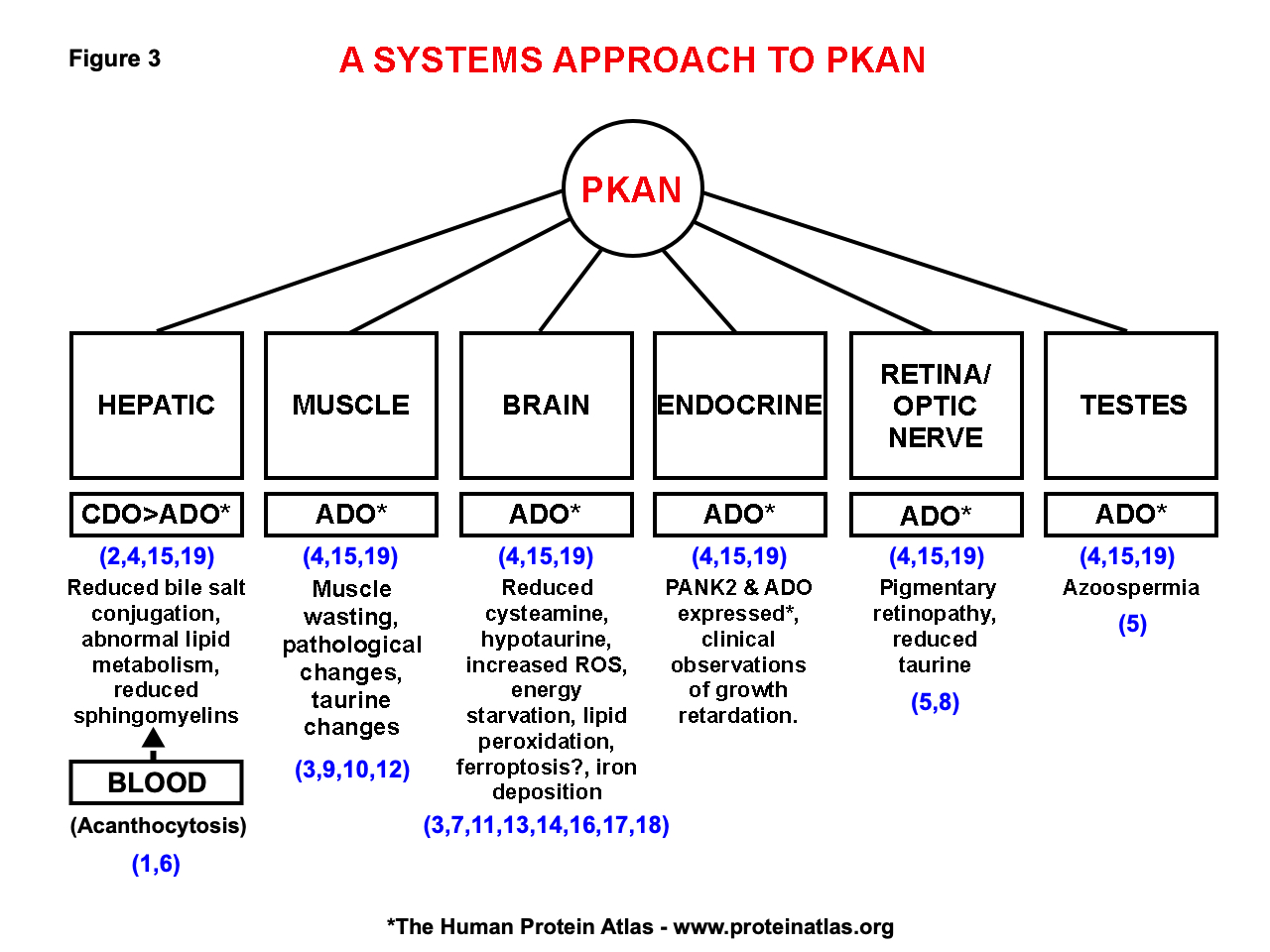
Background: PKAN is a recessive, rare, inborn error with an incidence of 1-3 per million, basal ganglia iron accumulation and a defect in PANK2 gene, the rate-limiting enzyme in coenzyme A synthesis. Many genes exist for PKAN, but RNA sequencing has not been systematically examined. A large PKAN cohort (70 patients, c.680 A>G mutation) in the Dominican Republic (DR) shows prevalence 1,000 times higher than normal; 1-2 patients/1000, and a carrier rate of 15%.The traditional view of the CoA pathway in PKAN (fig 1) fails to explain complex pathways involved in PANK2 gene function and the body systems involved. Our model incorporates cysteine, cysteamine, ADO, and CDO (fig 2). Clinical symptoms involve hepatic, brain, muscle, blood, and eye systems in this disorder (fig 3). Altered hepatic function reduces bile salt conjugation and changes lipid metabolism; changes in lipid metabolism alter red cell membranes with formation of acanthocytes. Brain pathology includes iron accumulation and cell death. Muscle involvement include wasting and pathologic features, and eye changes include retinopathy. Our model includes pantetheine and pantetheine kinase as a therapeutic entry point into the cycle (fig 2). Clinically, two DR patients receiving pantethine had significant improvement (18); 6 additional patients show improvement and treatment is ongoing. Pantethine rapidly converts to pantetheine, a substrate of pantetheine kinase. Pantetheine hydrolyzes into cysteamine and pantothenate; cysteamine enters the cycle via ADO (fig 2).
Method: We will treat 20 genetically confirmed patients using oral pantethine. Evaluation of pre- and post-treatment include BMI, BMR, fat and muscle mass, video scoring, MRI, blood lipids, bile salts, and acanthocytes, retinal exams, and RNA sequencing.
Results: Treatment effects will be measured as quantifiable changes in symptoms, metabolism, and genetic markers that occur in our PKAN patients. The detailed protocol will be presented.
Conclusion: Our systems approach model for PKAN helps to explain complexity of the metabolic pathways involved in PANK2 gene function, the myriad of body systems involved, and supports the use of pantethine in the treatment of PKAN.
References: 1. Leoni V, Strittmatter L, Zorzi G, et al. Metabolic consequences of mitochondrial coenzyme A deficiency in patients with PANK2 mutations. Mol Gen Metab 2012; 105(3), 463–471. 2. Stipanuk MH, Simmons CR, Andrew Karplus P, et al. Thiol dioxygenases: unique families of cupin proteins. Amino Acids 2010; 41(1), 91–102. 3. Brunetti D, Dusi S, Giordano C, et al. Pantethine treatment is effective in recovering the disease phenotype induced by ketogenic diet in a pantothenate kinase-associated neurodegeneration mouse model. Brain 2013; 137(1), 57–68. 4. Dominy JE, Simmons CR, Hirschberger LL, Hwang J, Coloso RM, Stipanuk MH. Discovery and Characterization of a Second Mammalian Thiol Dioxygenase, Cysteamine Dioxygenase. J Biol Chem 2007; 282(35), 25189–25198. 5. Kuo YM, Duncan JL, Westaway SK, et al. Deficiency of pantothenate kinase 2 (Pank2) in mice leads to retinal degeneration and azoospermia. Hum Mol Gen 2004; 14(1), 49–57. 6. Schiessl-Weyer J, Roa P, Laccone F, et al. Acanthocytosis and the c.680 A>G Mutation in the PANK2 Gene: A Study Enrolling a Cohort of PKAN Patients from the Dominican Republic. PLOS ONE 2015; 10(4), e0125861. 7. Brunetti D, Dusi S, Morbin M, et al. Pantothenate kinase-associated neurodegeneration: altered mitochondria membrane potential and defective respiration in Pank2 knock-out mouse model. Hum Mol Gen 2012; 21(24), 5294–5305. 8. Han J, Kim DW, Lee CH, Han SH. Optic Atrophy in a Patient With Atypical Pantothenate Kinase-Associated Neurodegeneration. J Neuro-Ophthalmology 2016; 36(2), 182–186. 9. Malandrini A, Bonuccelli U, Parrotta E, et al. Myopathic involvement in two cases of Hallervorden-Spatz disease. Brain and Development 1995; 17(4), 286–290. 10. De Luca A, Pierno S, Camerino DC. Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J Transl Med 2015;13(1). 11. Nishimura T, Higuchi K, Yoshida Y, et al. Hypotaurine Is a Substrate of GABA Transporter Family Members GAT2/Slc6a13 and TAUT/Slc6a6. Biol Pharm Bull 2018; 41(10), 1523–1529. 12.Warskulat U, Flogel U, Jacoby C, et al. Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J 2004; 18(3), 577–579. 13. Rodriguez-Raecke R, Roa-Sanchez P, Speckter H, et al. Grey matter alterations in patients with Pantothenate Kinase-Associated Neurodegeneration (PKAN). Parkinsonism & Related Disorders 2014; 20(9), 975–979. 14. Roa P, Bido P, Foerster B, et al. Evaluation of the “Tiger’s Eye” by Quantitative Susceptibility Imaging. ARC J Rad Med Imag 2017; Vol 2 (2), 7-11. 15. Gout, I. Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem Soc Trans 2018; 46(3), 721–728. 16. Aruoma OI, Halliwell B, Hoey B, et al. The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem J 1988; 256(1), 251–255. 17. Vitvitsky V, Garg SK, Banerjee R. Taurine Biosynthesis by Neurons and Astrocytes. J Biol Chem 2011; 286(37), 32002–32010. 18. Cruz Vicioso R, Roa P. Clinical Evolution of Patients with Pantothenate Kinase‐associated Neurodegeneration after Therapy with Pantethine (vitamin b5): Report of Two Cases. Mov Disorders 2018; Vol 33 (S1), 165. 19. Novelli GD. Enzymatic synthesis and structure of CoA. Fed Proc 1953; 12 (3), 675–81.
To cite this abstract in AMA style:
S. Baser, C. Muniz, F. Middleton, R. Ericson, C. Bass. A Systems Approach Model for Pantothenate Kinase-Associated Neurodegeneration (PKAN)- Assessment and Treatment [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/a-systems-approach-model-for-pantothenate-kinase-associated-neurodegeneration-pkan-assessment-and-treatment/. Accessed February 6, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-systems-approach-model-for-pantothenate-kinase-associated-neurodegeneration-pkan-assessment-and-treatment/