Category: Parkinson’s Disease: Clinical Trials
Objective: To determine whether faecal microbiota transplant (FMT) improves motor function in patients with Parkinson Disease (PD) treated with levodopa/carbidopa intestinal gel (LCIG).
Background: Preliminary findings from case reports and studies in human subjects suggest that FMT has therapeutic potential in PD with positive effects on both motor and non-motor symptoms (1,2,3).
Method: We conducted a randomised, blinded, placebo-controlled, crossover trial of FMT in patients with PD already on treatment with LCIG. Patients were recruited if they were receiving LCIG via percutaneous endoscopic jejunostomy tube on a stable dose for 6 months, had ≥ 3 hours of OFF time. Patients were randomized 1:1 to FMT or Placebo. After randomisation, patients received their infusions via their jejunal tube at visit 2 and then again two weeks later at visit 3. A four-week washout period then occurred after which patients crossed over and received their infusions at visit 4 and then again two weeks later at visit 5. Patients, outcome assessors and statisticians were blinded to randomization. The primary outcomes are 1) safety and tolerability of FMT infusion via PEJ tube, based on clinical assessment during the infusion, patient report during the infusion and 48 hours post-infusion; and 2) change in daily OFF time as measured by the Hauser diary. Secondary outcomes included constipation and PD scales and quality of life scores.
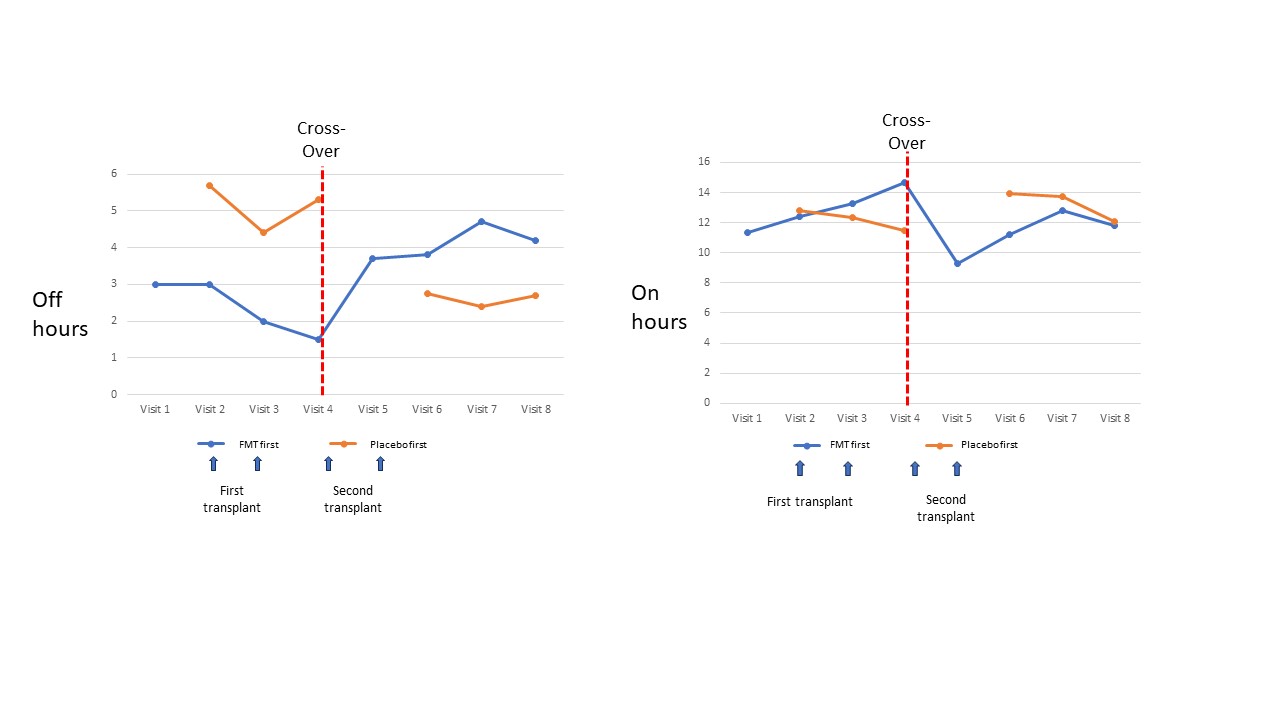
Results: We undertook a preliminary analysis after recruiting nine patients. The median age of the patients was 64 and 22% were female. There were no serious adverse events during the study period. There were three infusion-related events where blood pressure fluctuated by >20% in three patients but all were self-limiting and did not require medical attention. Five patients experienced mild abdominal discomfort and bloating after their infusions: two patients after FMT, two after Placebo and one patient after both Placebo and FMT infusions. When compared with Placebo, those treated with FTM had an absolute reduction in 1.04 OFF hours (95% CI -2.99 to 0.9, p=0.193) and an absolute increase in ON hours by 1.53 (95% CI -0.35 to 3.05, p=0.113) (figure 1).
Conclusion: This preliminary analysis shows FMT transplant in PD patients receiving LCIG is a safe and potentially effective treatment. The study is ongoing.
Figure 1
References: 1. Sampson TR, Debelius JW, Thron T, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016; 167: 1469-1480.e12.
2. Huang H, Xu H, Luo Q, et al. Fecal microbiota transplantation to treat Parkinson’s disease with constipation: A case report. Medicine (United States); 98. Epub ahead of print June 1, 2019. DOI: 10.1097/MD.0000000000016163.
3. Vendrik KEW, Ooijevaar RE, de Jong PRC, et al. Fecal Microbiota Transplantation in Neurological Disorders. Frontiers in Cellular and Infection Microbiology; 10. Epub ahead of print March 24, 2020. DOI: 10.3389/fcimb.2020.00098.
To cite this abstract in AMA style:
D. Wilson, L. Williams, S. Waller, S. Dal, J. Qiu, D. Tsui, S. Bray, J. Griffith, D. Galea, X. Chen, F. Tudehope, L. Bracken, H. Morales-Briceno, N. Mahant, F. Chang, S. Bandodkar, D. Van-Der-Poorten, V. Fung. A Pilot Study of Intrajejunal Faecal Microbiota Transplant to Improve Motor Function in Patients with Parkinson’s Disease Treated with Levodopa-carbidopa Intestinal Gel: Preliminary Findings. [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/a-pilot-study-of-intrajejunal-faecal-microbiota-transplant-to-improve-motor-function-in-patients-with-parkinsons-disease-treated-with-levodopa-carbidopa-intestinal-gel-preliminary-findings/. Accessed April 20, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-pilot-study-of-intrajejunal-faecal-microbiota-transplant-to-improve-motor-function-in-patients-with-parkinsons-disease-treated-with-levodopa-carbidopa-intestinal-gel-preliminary-findings/