Objective: Spastic paraplegia type 55 (SPG55) is an autosomal recessive complicated HSP caused by homozygous mutation in the C12orf65 gene (613541) on chromosome 12q24. The severity of symptoms and progression rate are quite variable. We attempt to identify the new mutation of the C12orf65 gene in a Chinese HSP pedigree and explore the potential pathogenesis.
Background: Spastic paraplegia type 55 is rare reported in China, as an autosomal recessive complicated hereditary spastic paraplegia (HSP), resulting from homozygous mutations in the C12orf65 gene, which encodes a mitochondrial matrix protein. We attempt to identify the new mutation of the C12orf65 gene in a Chinese HSP pedigree and explore the potential pathogenesis.
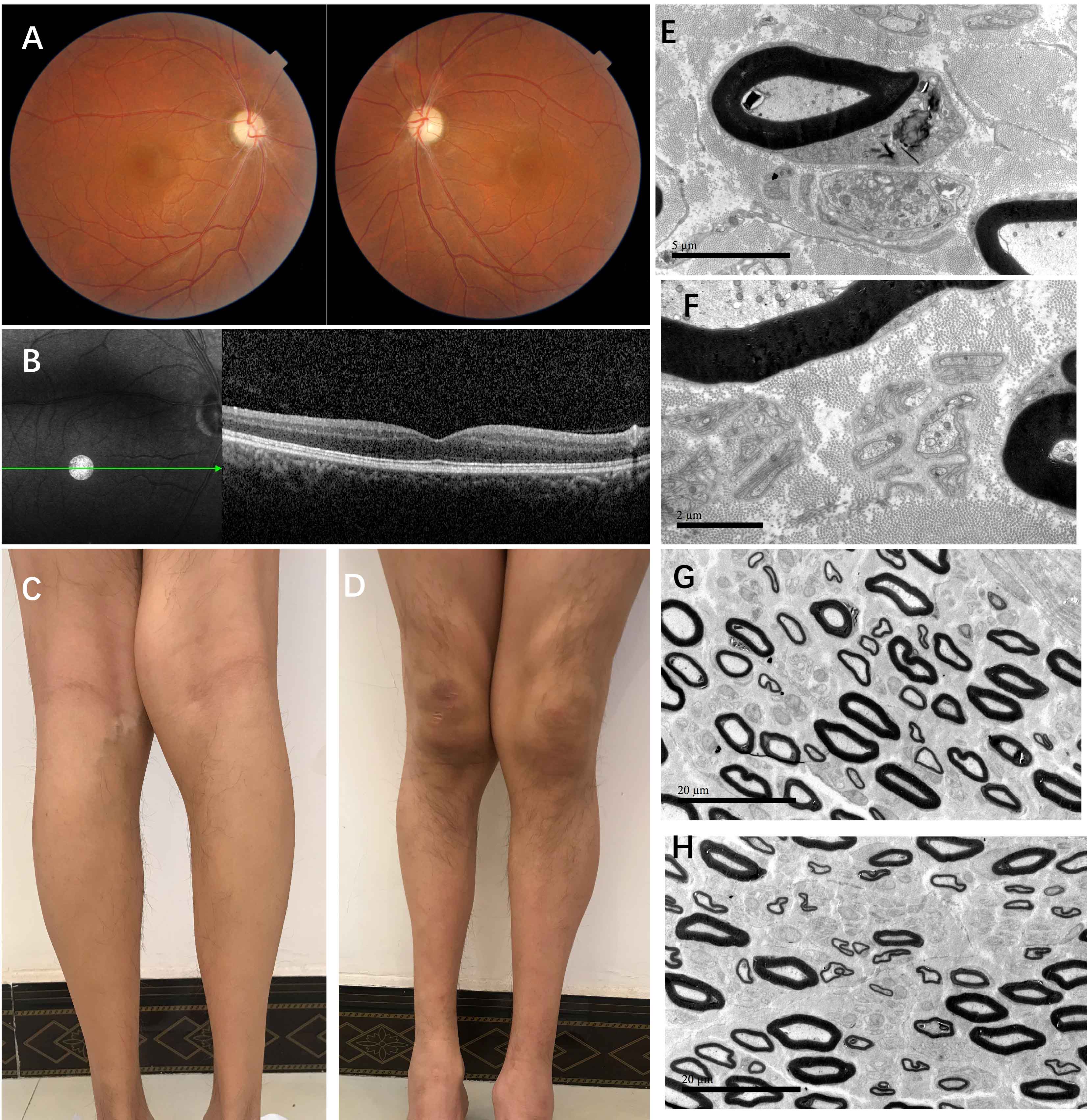
Method: Here we presented a young patient in an autosomal recessive inheritance with optic atrophy, lower extremity spasticity and distal progressive peripheral neuropathy. DNA analysis was conducted for the patient and his family. Neurologic examination, funduscopic examination, nerve conduction, sural nerve biopsy was performed for the patient. Cell biology experiments were conducted in fibroblasts by skin biopsy from the patient.
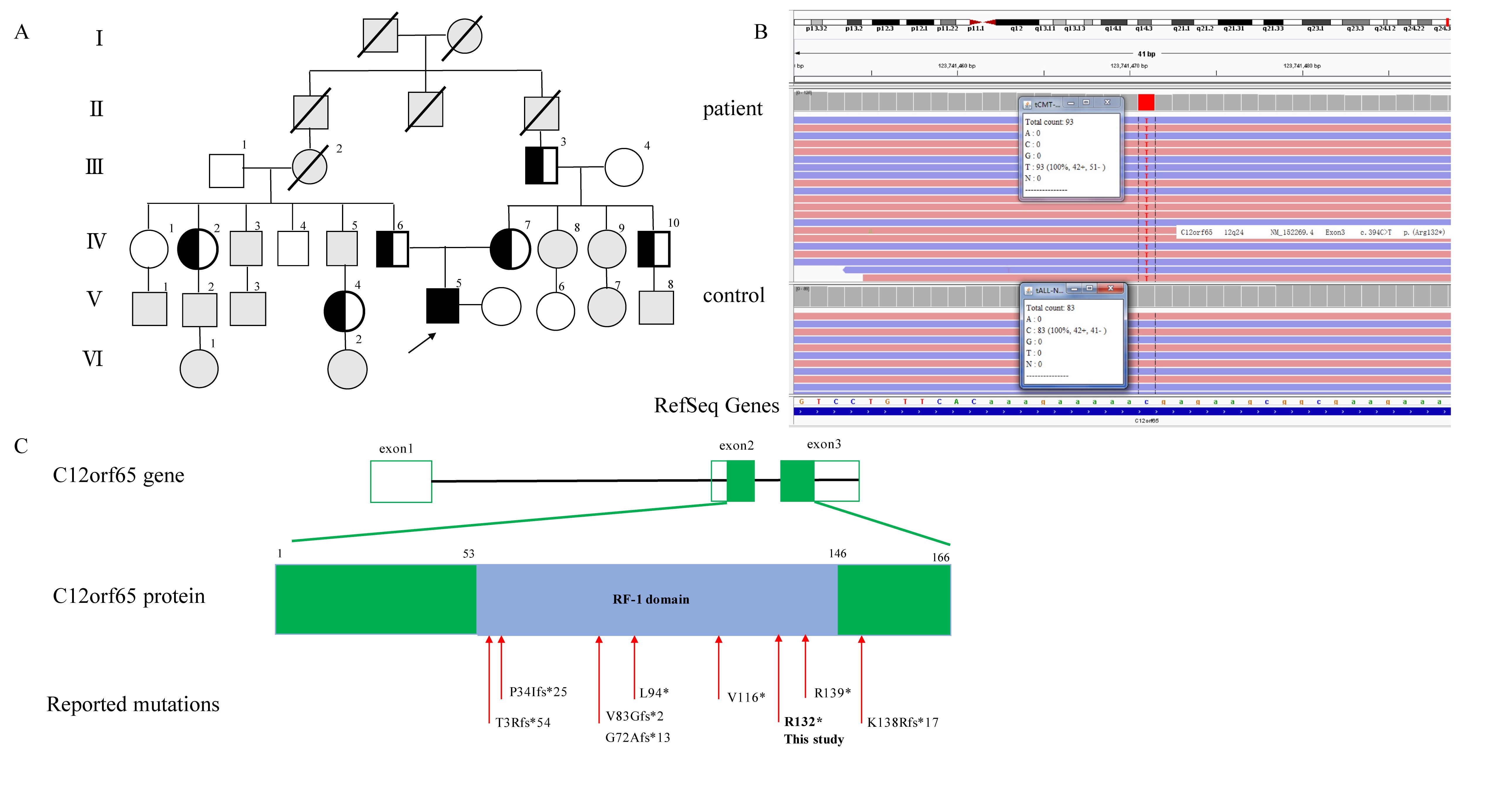
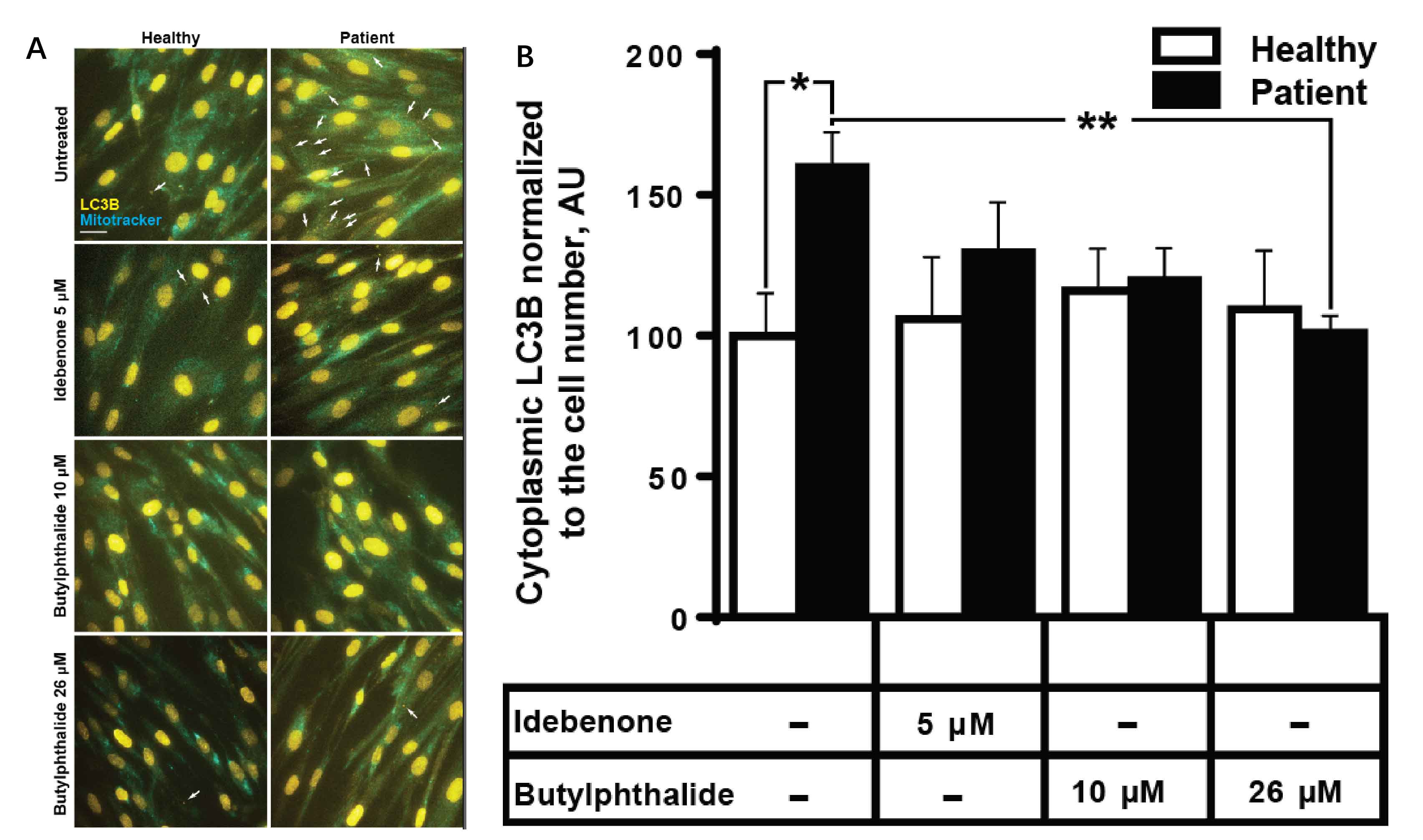
Results: We detected a homozygous C12orf65 nonsense mutation (c.394C>T, p.R132X) in the patient. The novel truncated mutation of C12orf65 was first described in China. The clinical manifestation showed optic atrophy with reduced visual acuity, lower limb spasticity with hyperreflexia and anterior tibial muscle weakness and atrophy. Funduscopic examination showed that the optic nipples were pale in color and clear in borders, suggesting a significant atrophy of the optic nerve. Nerve conduction was consistent with a demyelinating sensory and motor neuropathy. A chronic predominantly hypomyelinating neuropathy were revealed by sural nerve biopsy. Through the cell biology experiments, we observed LC3B, the protein crucial for autophagy, was up-regulated in fibroblasts from C12orf65-deficient patients and was normalized when treated by Dl-3-N-butylphthalide with 26 µM.
Conclusion: The novel nonsense mutation of C12orf65 could cause AR-HSP with optic atrophy, progressive distal lower extremity spasticity and bilateral lower limb muscle atrophy. The autophagy pathway may be involved in the pathogenesis of HSP 55.
References: 1. Souza D, Paulo V S, et al. “Hereditary Spastic Paraplegia: Clinical and Genetic Hallmarks” [J].The Cerebellum,2017,16 (2):525-551 2. Kumar K R, Blair N F, et al. An Update on the Hereditary Spastic Paraplegias: New Genes and New Disease Models[J]. Movement Disorders Clinical Practice, 2015, 2(3):213-223. 3. Antonicka H, Stergaard E, et al. Mutations in C12orf65 in Patients with Encephalomyopathy and a Mitochondrial Translation Defect[J]. American Journal of Human Genetics, 2010, 87(1):115-122. 4. Shimazaki H, Takiyama Y, et al. A homozygous mutation of C12orf65 causes spastic paraplegia with optic atrophy and neuropathy (SPG55)[J]. Journal of Medical Genetics, 2012, 49(12):777-784. 5. Chang J , Lee S , et al. Spastic paraplegia proteins spastizin and spatacsin mediate autophagic lysosome reformation[J]. Journal of Clinical Investigation, 2014, 124(12):5249-5262. 6. Hardie, Grahame D. AMP-Activated Protein Kinase as a Drug Target[J]. Annual Review of Pharmacology and Toxicology, 2007, 47(1):185-210. 7. Vingtdeux V, Chandakkar P, et al. Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-? peptide degradation[J]. The FASEB Journal, 2011, 25(1):219-231. 8. Kim J , Kundu M , et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1[J]. NATURE CELL BIOLOGY, 2011, 13(2):132-141. 9. Hoeffer C A , Sanchez E , et al. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome[J]. Genes, brain, and behavior, 2012, 11(3):332-341. 10. Narendra D P, Jin S M, et al. PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin[J]. PLoS Biology, 2010, 8(1):315-316. 11. Evelyn S. Vincow, Gennifer Merrihew, Ruth E. Thomas, Nicholas J. Shulman, Richard P. Beyer, Michael J. MacCoss, and Leo J. Pallanck. The PINK1–Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. PNAS April 16, 2013 110 (16) 6400-6405; 12. Zhang C, Yu X, et al. PINK1/Parkin-mediated mitophagy was activated against 1,4-enzoquinone-induced apoptosis in HL-60 cells[J]. Toxicology in Vitro, 2018,50:217-224. 13. Li H, Durbin R.Fast and accurate long-read alignment with Burrows-Wheeler transform[J]. Bioinformatics,2010, 26(5): 589–595. 14. Lu Zh, Jing Zh, et al. PriVar: a toolkit for prioritizing SNVs and indels from next-generation sequencing data[J]. Bioinformatics,2012, 29(1):124-125. 15. Buchert R , Uebe S , et al. Mutations in the mitochondrial gene C12ORF65 lead to syndromic autosomal recessive intellectual disability and show genotype phenotype correlation[J]. European Journal of Medical Genetics, 2013, 56(11):599-602. 16. Spiegel R , Mandel H , et al. Delineation of C12orf65-related phenotypes: a genotype–phenotype relationship[J]. European Journal of Human Genetics, 2014, 22(8):1019-1025. 17. Tucci A , Liu Y T , Preza E , et al. Novel C12orf65 mutations in patients with axonal neuropathy and optic atrophy[J]. Journal of Neurology, Neurosurgery & Psychiatry, 2014, 85(5):486-492. 18. Pyle A , Ramesh V , Bartsakoulia M , et al. Behr’s Syndrome is Typically Associated with Disturbed Mitochondrial Translation and Mutations in the C12orf65 Gene[J]. Journal of Neuromuscular Diseases, 2015, 1(1):55-63. 19. Maria W , Gorman G S , et al. Adult Onset Leigh Syndrome in the Intensive Care Setting: A Novel Presentation of a C12orf65 Related Mitochondrial Disease[J]. Journal of Neuromuscular Diseases, 2015, 2(4):409-419. 20. Imagawa E , Fattal-Valevski A , et al. Homozygous p.V116* mutation in C12orf65 results in Leigh syndrome[J]. Journal of Neurology, Neurosurgery & Psychiatry, 2015:jnnp-2014-310084. 21. Nishihara H, Omoto M, et al. Autopsy case of the C12orf65 mutation in a patient with signs of mitochondrial dysfunction[J]. Neurol Genet. 2017;3(4):e171. 22. Fang XJ, Zhang W, et al. Compound Heterozygote Mutation of C12orf65 Causes Distal Motor Neuropathy and Optic Atrophy[J]. Chin Med J (Engl). 2017;130(2):242–244. 23. Holbrook JA, Neu-Yilik G, et al. Nonsense-mediated decay approaches the clinic. [J] Nat Genet 2004;36:801e8. 24. Khajavi M, Inoue K, et al. Nonsense-mediated mRNA decay modulates clinical outcome of genetic disease[J]. Eur J Hum Genet 2006;10:1074e81. 25. Oz-Levi D, Ben-Zeev B, Ruzzo EK, et al. Mutation in TECPR2 reveals a role for autophagy in hereditary spastic paraparesis[J]. Am J Hum Genet 2012; 91:1065-1072. 26. Vantaggiato C, Crimella C, Airoldi G, et al. Defective au- tophagy in spastizin mutated patients with hereditary spastic paraparesis type 15[J]. Brain 2013; 136:3119-3139. 27. Zhang T , Wang H , et al. Modulating autophagy affects neuroamyloidogenesis in an in vitro ischemic stroke model[J]. Neuroscience, 2014, 263:130-137. 28. Ying Peng, Yanli Hu, et al. L-3-n-butylphthalide Reduces Tau Phosphorylation and Improves Cognitive Deficits in A beta PP/PS1-Alzheimer’s Transgenic Mice[J]. Journal of Alzheimers Disease Jad, 2012, 29(2):379-391.
To cite this abstract in AMA style:
L. Wu, Y. Xu, Q. Wang, X. Lai, I. Vinnikov, W. Chen. A C12orf65 mutation-related autosomal recessive hereditary spastic paraplegia is associated with autophagy induction [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/a-c12orf65-mutation-related-autosomal-recessive-hereditary-spastic-paraplegia-is-associated-with-autophagy-induction/. Accessed April 18, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-c12orf65-mutation-related-autosomal-recessive-hereditary-spastic-paraplegia-is-associated-with-autophagy-induction/