Session Information
Date: Tuesday, June 21, 2016
Session Title: Parkinson's disease: Pathophysiology
Session Time: 12:30pm-2:00pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To investigate the potential role of macroautophagy in the regulation of microglia-mediated neuroinflammation and dopamine neuron injury.
Background: Chronic neuroinflammation, a process mediated by persistent microglia activation and overproduction of inflammatory mediator is involved in the pathogenesis of Parkinson’s disease (PD). Among various inflammatory molecules, tumor necrosis factor-α has been implicated as a major mediator of neurodegeneration in PD and the plasma TNF-α level correlated with the non-motor symptoms of PD. It has been reported that loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. However, how autophagy activation modulates microglial inflammatory profiles remains unclear.
Methods: In TNF-α-treated microglia, Western blotting analysis was applied to examine the protein expressions of autophagy-related markers including LC3, p62 and Beclin; Quantitative and reverse transcription PCR were used to detect M1 and M2 phenotypic marker changes in mRNA levels; Upregulation or inhibition of autophagy then observe the mRNA changes; The primary microglial cell culture supernatant (microglia conditioned medium) and neurons culture medium were mixed at the ratio of 1: 2 after that the the mixed medium were added in primary neurons co-cultured for 24h, CCK8 and immunofluorescence were used to detect the different groups viability of primary midbrain neurons and changes in the projections of neurons.
Results: TNF-α treatment caused significant increases in autophagy-related proteins LC3, p62 and Beclin1 and the M1 marker (iNOS, IL-1β) increased while the M2 marker (Arginase,Ym1/2) decreased (figure1). In the inflammation model, upregulation of autophagy (rapamycin, resveratrol, serum deprivation) decreased the M1 marker but increased the M2 marker. Inhibition of autophagy (3-MA,Atg5 knockout) produced opposite effects.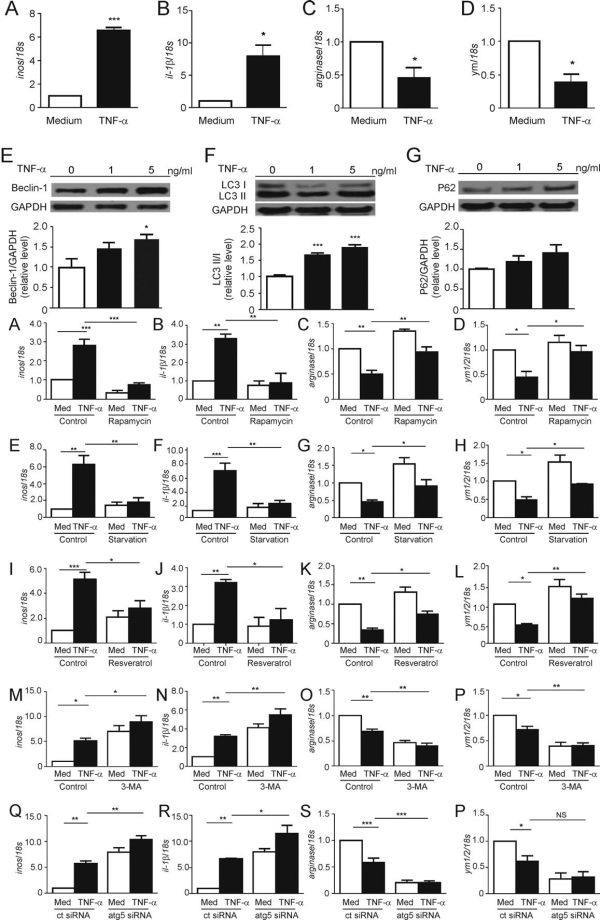 The CCK8 assay revealed that co-cultured with TNF-α-treated microglia medium, the neurons viability decreased. Inhibition of autophagy in microglia culture supernatants further decreased the viability of neurons and compared with model group (figure2). MAP-2 immunostaining displayed that inhibition of autophagy in microglia caused the neurites shortening of MAP-2 positive neurons.
The CCK8 assay revealed that co-cultured with TNF-α-treated microglia medium, the neurons viability decreased. Inhibition of autophagy in microglia culture supernatants further decreased the viability of neurons and compared with model group (figure2). MAP-2 immunostaining displayed that inhibition of autophagy in microglia caused the neurites shortening of MAP-2 positive neurons.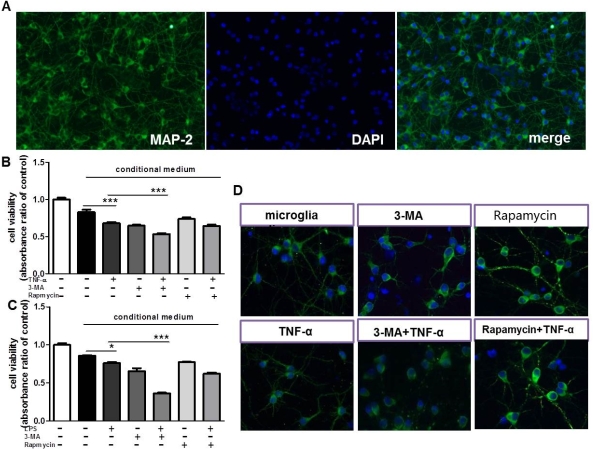
Conclusions: Macorautophagy can regulate microglia-mediated neuroinflammation, and the enhancement of autophagy can promote the microglia polarization toward M2 phenotype.
To cite this abstract in AMA style:
M. Jin, L. Hu, C. Liu. Role of macroautophagy in regulating microglia-mediated neuroinflammation and dopaminergic neuron injury [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/role-of-macroautophagy-in-regulating-microglia-mediated-neuroinflammation-and-dopaminergic-neuron-injury/. Accessed March 31, 2025.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/role-of-macroautophagy-in-regulating-microglia-mediated-neuroinflammation-and-dopaminergic-neuron-injury/
