Objective: To evaluate the efficacy and safety of controlled-release levodopa (CRLD) compared with standard release levodopa (SRLD) in patients with Parkinson’s Disease (PD) and motor fluctuations.
Background: Levodopa (LD) is the most effective treatment for PD, but over the years of treatment, PD patients start to present dyskinesias and motor fluctuations, showing a diminished benefit in advanced stages of disease [1,2]. Although it is plausible to use CRLD formulations in early and late stage PD patients [1-3], there is no definitive evidence of its superiority to SRLD[1].
Method: We systematically reviewed PUBMED, EMBASE and Cochrane Library databases for randomized controlled trials (RCTs) with PD patients presenting motor fluctuations treated with CRLD. Outcomes of interest were off-time, on-time with troublesome dyskinesia, on-time without dyskinesia and adverse events. Statistical analysis was performed in Review Manager 5.4.1. [4], and heterogeneity was assessed with I2. We considered crossover studies as if they were parallel-group trials for our analysis.
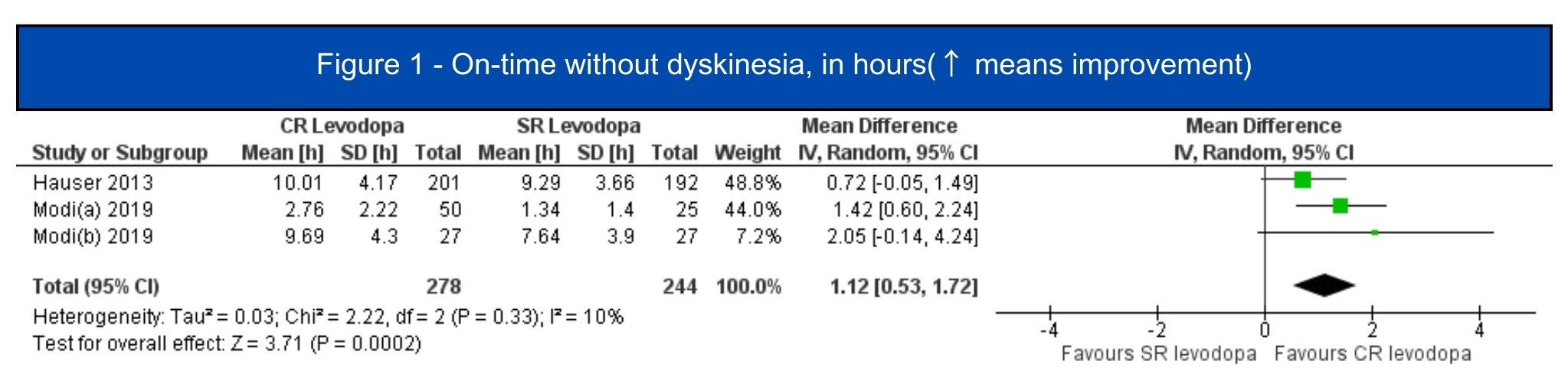
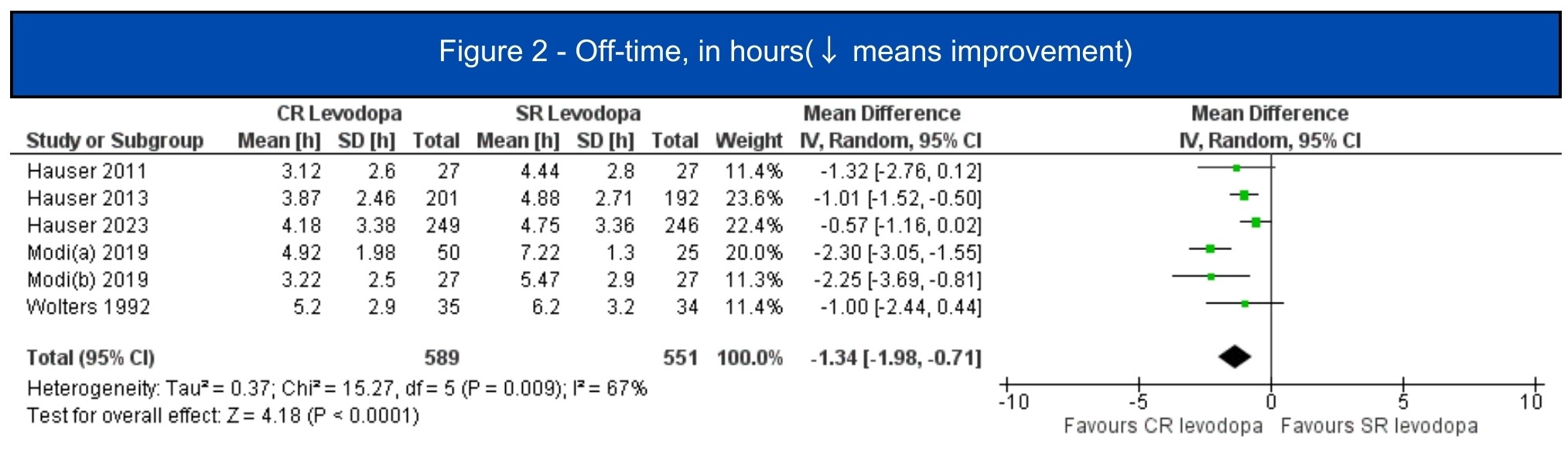
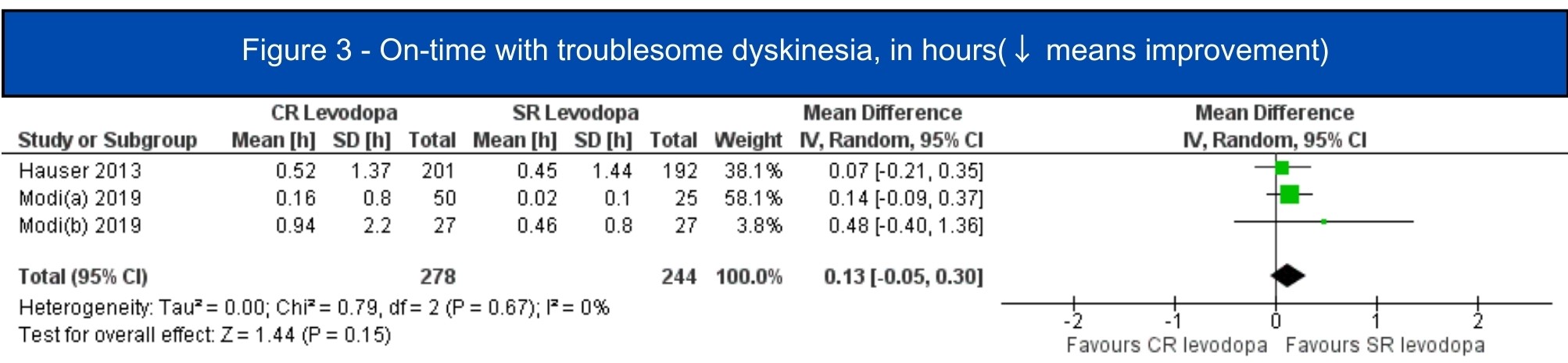
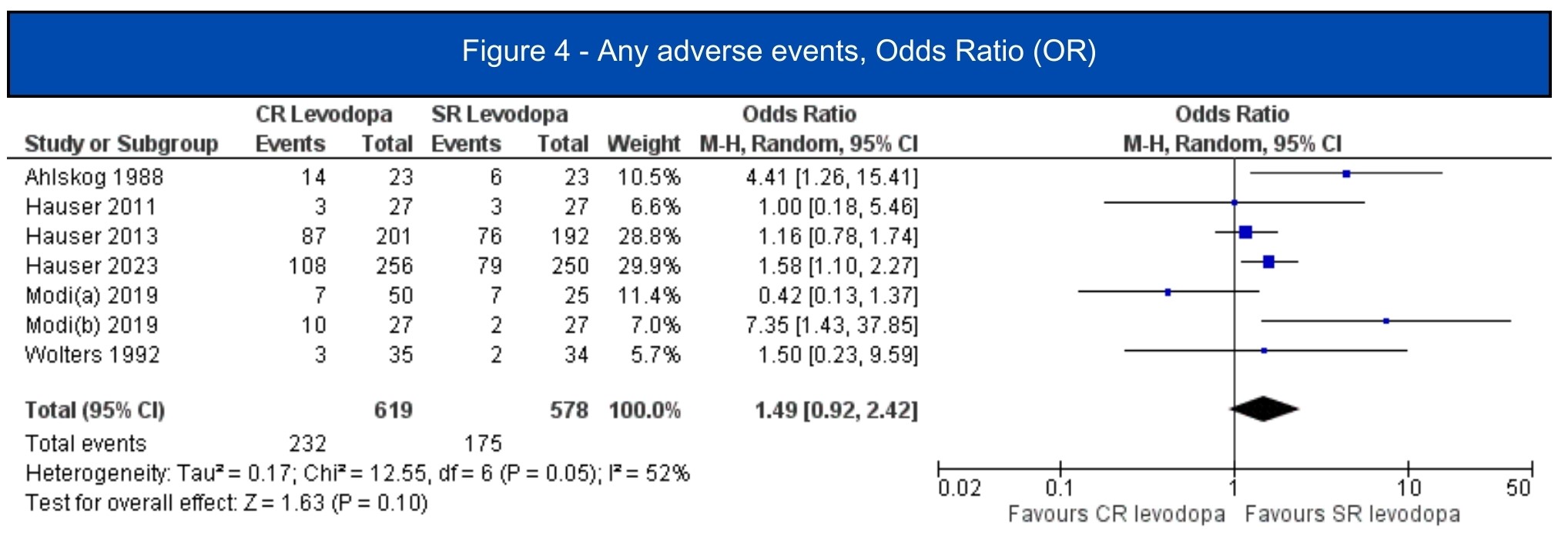
Results: We included 7 studies (1.197 patients, 619 in the CRLD group)[5-11]. All included studies were RCTs, four of them were crossover studies[6-8,11]. In the pooled analysis, CRLD was associated with an increase in daily on-time without dyskinesia (MD 1.12; 95% CI 0.53-1.72; I2=10%; p=.0002) [5-7][figure1] and a decrease in off-time (MD -1.34; 95% CI -1.98 – -0.71; I2=67%; p<.0001) [5-10] [figure2] when compared to SRLD. Alternatively, on-time with troublesome dyskinesia [5-7] and the occurrence of any adverse event [5-11] had a tendency to worsen with CRLD, but the result was non-significant [figure3, figure4].
Conclusion: In conclusion, our analysis suggests that CRLD is superior to SRLD in improving off-time and on-time without dyskinesia, leading to a better control of PD disease, without significantly increasing adverse events. Due to treating crossover studies as parallel-groups, we incurred a unit-of-analysis error, with potential to widen the confidence intervals and underpower those studies; however, we found statistically significant results despite this possibility. Finally there is still a need for future studies that could ascertain if this improvement in off and on-time translates into better quality of life for PD patients.
Figure 1 – On-time without dyskinesia
Figure 2 – Off-time
Figure 3 – On-time with troublesome dyskinesia
Figure 4 – Any Adverse Events
References: [1] Saba RA, Maia DP, Cardoso FEC, Borges V, F. Andrade LA, Ferraz HB, et al. Guidelines for Parkinson’s disease treatment: consensus from the Movement Disorders Scientific Department of the Brazilian Academy of Neurology – motor symptoms. Arq Neuro-Psiquiatr. 2022 Mar;80(3):316–29.
[2] Fabbrini G, Stasio F, Bloise M, Berardelli A. Soluble and controlled-release preparations of levodopa: do we really need them? J Neurol. 2010 Nov;257(S2):292–7.
[3] Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020 Feb 11;323(6):548.
[4] Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020.
[5] Hauser RA, Hsu A, Kell S, Espay AJ, Sethi K, Stacy M, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson’s disease and motor fluctuations: a phase 3 randomised, double-blind trial. The Lancet Neurology. 2013 Apr;12(4):346–56.
[6] Modi NB, Mittur A, Rubens R, Khanna S, Gupta S. Single-Dose Pharmacokinetics and Pharmacodynamics of IPX203 in Patients With Advanced Parkinson Disease: A Comparison With Immediate-Release Carbidopa-Levodopa and With Extended-Release Carbidopa-Levodopa Capsules. Clin Neuropharm. 2019 Jan;42(1):4–8.
[7] Modi NB, Mittur A, Dinh P, Rubens R, Gupta S. Pharmacodynamics, Efficacy, and Safety of IPX203 in Parkinson Disease Patients With Motor Fluctuations. Clin Neuropharm. 2019 Sep;42(5):149–56.
[8] Hauser RA, Ellenbogen AL, Metman LV, Hsu A, O’Connell MJ, Modi NB, et al. Crossover comparison of IPX066 and a standard levodopa formulation in advanced Parkinson’s disease. Movement Disorders. 2011 Oct;26(12):2246–52.
[9] Hauser RA, Espay AJ, Ellenbogen AL, Fernandez HH, Isaacson SH, LeWitt PA, et al. IPX203 vs Immediate-Release Carbidopa-Levodopa for the Treatment of Motor Fluctuations in Parkinson Disease: The RISE-PD Randomized Clinical Trial. JAMA Neurol. 2023 Oct 1;80(10):1062.
[10] Wolters EC, Horstink MWIM, Roos RAC, Jansen ENH. Clinical efficacy of sinemet CR 50/200 versus sinemet 25/100 in patients with fluctuating parkinson’s disease. Clinical Neurology and Neurosurgery. 1992 Sep;94(3):205–11.
[11] Ahlskog JE, Muenter MD, McMANIS PG, Bell GN, Bailey PA. Controlled-Release Sinemet (CR-4): A Double-Blind Crossover Study in Patients With Fluctuating Parkinson’s Disease. Mayo Clinic Proceedings. 1988 Sep;63(9):876–86.
To cite this abstract in AMA style:
G. Bolner, Y. Rossi, E. de Almeida, F. de Oliveira, V. Müller, L. Barbosa Lima, A. Rossato, G. Guindani Maia, A. Hilbig. Efficacy and Safety of Controlled-Release Levodopa in Parkinson’s Disease: a Systematic Review and Meta-Analysis [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-controlled-release-levodopa-in-parkinsons-disease-a-systematic-review-and-meta-analysis/. Accessed March 1, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-controlled-release-levodopa-in-parkinsons-disease-a-systematic-review-and-meta-analysis/