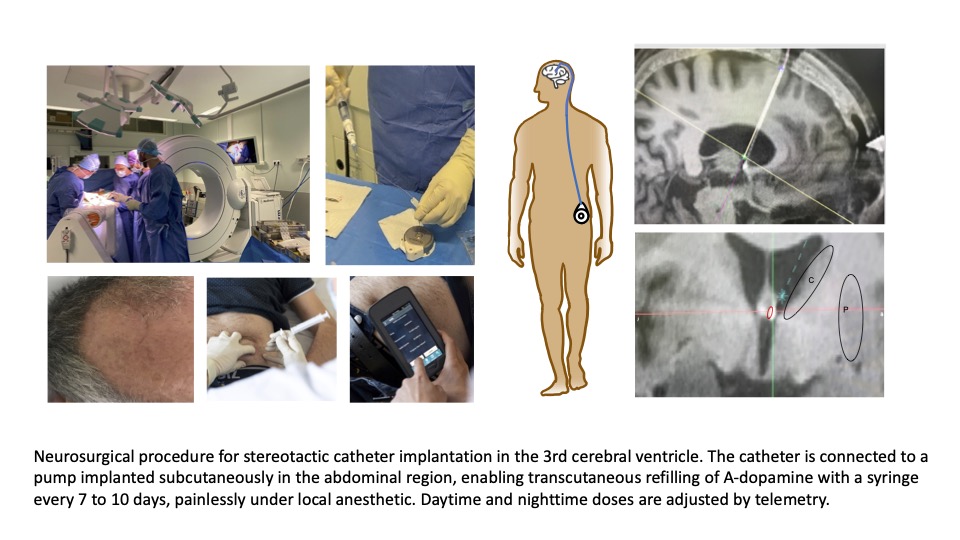
Category: Parkinson’s Disease: Clinical Trials
Objective: To assess the continuous intracerebroventricular (i.c.v.) of A-dopamine in patients with a telemetry-controlled intra-abdominal pump system connected to a subcutaneous catheter implanted through the right frontal horn into the third ventricle, close to the striatum.
Background: Dopamine does not cross the digestive or blood-brain barriers. L-dopa has numerous pharmacokinetic and pharmacodynamic drawbacks, leading to L-dopa-related complications (LDRC). Continuous circadian compensation of dopamine deficiency in the nigro-striatal pathway represents an ideal treatment for PD. To prevent dopamine oxidation, a novel concept of continuous i.c.v. administration of an anaerobic dopamine formulation (A-dopamine) has proved safe and effective in two rodent models and in a chronic non-human primate model of PD.
Method: We conducted a phase 1 study of A-dopamine titration with concomitant reduction of oral dopaminergic therapy followed by a randomized, controlled, open-label, crossover phase 2 study of one month of A-dopamine versus one month of best oral medical therapy. The primary endpoint in phase I was safety, and the primary endpoint in phase 2 was the blinded assessment of percentage over target (PTO, i.e., off and dyskinesia), recorded by actimetry at home using a wristwatch worn on the side most affected. Secondary endpoints included the evolution of ON and OFF fluctuations and dyskinesias on two-week home diaries, the Dyskinesia Rating Score (DRS) and MDS-UPDRS parts III and IV, safety and behavior using neuropsychological and psychiatric examinations.
Results: 12 participants were enrolled in phase 1, with no serious adverse events induced by A-dopamine. Two participants dropped out in phase 2 due to unrelated medical conditions. All individuals showed a significant reduction in PTO, off time and dyskinesias on home diaries, DRS and MDS-UPDRS parts III and IV on A-dopamine compared with the best medical treatment. Importantly, A-dopamine alone induced no dyskinesias and no adverse impact on behavior. All patients requested long-term treatment with a very good safety profile.
Conclusion: For the first time, we have demonstrated the clinical feasibility, safety and initial evidence of efficacy of A-dopamine neuromodulation in patients at the LDRC stage, motivating its clinical development in order to demonstrate the risk/benefit balance.
Therapeutic concept of A-dopamine
References: Preclinical validation in mouse and rat models: Laloux C, Gouel F, Lachaud C, et al. Continuous cerebroventricular administration of dopamine: A new treatment for severe dyskinesia in Parkinson’s disease? Neurobiol Dis 2017;103:24-31.
Preclinical validation in non human primate model: Moreau C, Rolland AS, Pioli E, et al. Intraventricular dopamine infusion alleviates motor symptoms in a primate model of Parkinson’s disease. Neurobiol Dis. 2020;139:104846.
To cite this abstract in AMA style:
C. Moreau, P. Odou, A. Demailly, G. Touzet, N. Reyns, J. Labreuche, A. Duhamel, C. Barthelemy, D. Lannoy, N. Carta, B. Palas, F. Marchand, B. Gouges, C. Leclerc, C. Potey, T. Ouk, K. Dujardin, S. Baigne, L. Carton, AS. Rolland, JC. Devedjian, V. Foutel, D. Deplanque, M. Fisichella, D. Devos. Pharmacological neuromodulation with intracerebroventricular administration of anaerobic dopamine for Parkinson’s disease [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/pharmacological-neuromodulation-with-intracerebroventricular-administration-of-anaerobic-dopamine-for-parkinsons-disease/. Accessed December 24, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/pharmacological-neuromodulation-with-intracerebroventricular-administration-of-anaerobic-dopamine-for-parkinsons-disease/