Category: Rating Scales
Objective: To evaluate and optimize the relevance, usability and longitudinal responsiveness of the Parkinson’s Disease-Health Index (PD-HI) for use in PD clinical trials.
Background: The use of valid, clinically relevant, and sensitive outcome measures is a priority of the FDA for PD therapeutic trials. In prior work, we developed and validated a disease-specific patient-reported outcome (PRO) measure known as the PD-HI. The present study is evaluating the PD-HI in a 24-month natural history study of PD to obtain longitudinal instrument metrics and optimize its current use for clinical trials and regulatory claims.
Method: We partnered with a variety of PD organizations (registries, foundations, support groups and clinics) to recruit an international cohort of adults with PD for a 24-month longitudinal study. Participants are completing the PD-HI, Neuro-QoL and the MDS-UPDRS parts 1b and 2 at baseline and every 6 months for a total of 24 months. Also, participants are completing a global impression of change questionnaire (6-24 months) and a survey preference form (baseline and 24 months). Statistical analysis will examine changes in PRO scores over time, the correlation of patient-reported disease progression with demographic and clinical characteristics and PD participant preferences for different PROs.
Results: 260 adults enrolled in the study and 206 participants completed at least one demographic survey question and one question on the PD-HI, meeting minimum threshold for data inclusion. A total of 149 participants completed all baseline survey assessments. To date, 114 participants have completed their 6-month assessment with all remaining 6-month assessments scheduled for completion by April 2024. Forthcoming longitudinal data will evaluate the PD-HI’s responsiveness to change and determine minimally clinically important difference values of the PD-HI and its subscales. This data will also assess how various PD symptomatic domains progress over time and identify which demographic and clinical characteristics are associated with a faster disease progression.
Conclusion: PD-HI is a 13-domain PRO that was designed and validated for use in PD clinical trials. Longitudinal evaluation of the PD-HI will further document its ability to track disease over time and quantify what constitutes a clinically meaningful change in its scores over time.
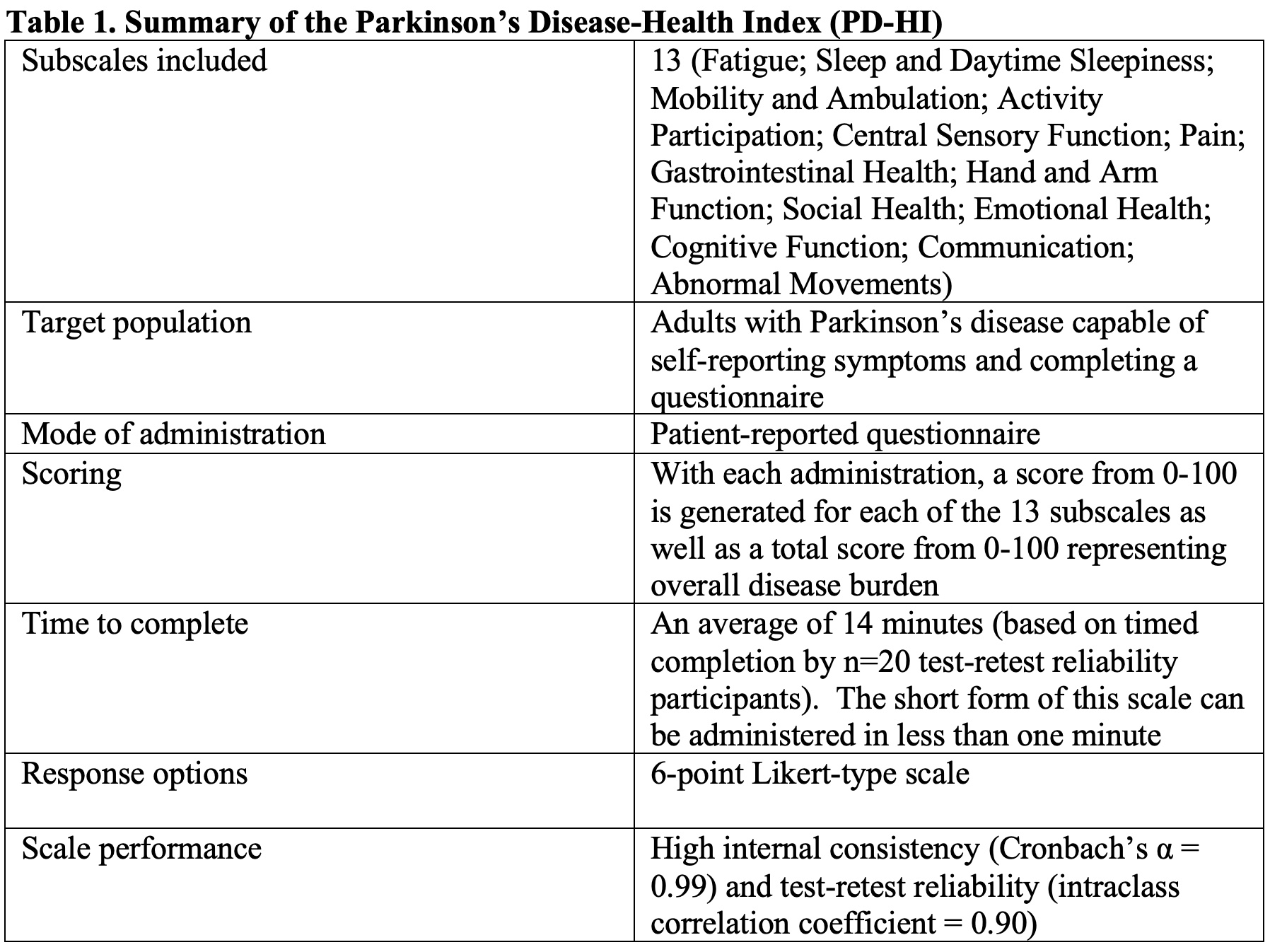
Summary of the PD-HI
To cite this abstract in AMA style:
J. Purks, J. Seabury, J. Weinstein, A. Varma, S. Rosero, C. Engebrecht, C. Irwin, C. Shupe, A. Arky, J. Heatwole, C. Zizzi, N. Dilek, J. Adams, E. Dorsey, C. Heatwole. Implementation of the Parkinson’s Disease-Health Index (PD-HI), a Novel, Validated, Disease-Specific Patient-Reported Outcome Measure, in a 24-Month Natural History Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/implementation-of-the-parkinsons-disease-health-index-pd-hi-a-novel-validated-disease-specific-patient-reported-outcome-measure-in-a-24-month-natural-history-study/. Accessed December 30, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/implementation-of-the-parkinsons-disease-health-index-pd-hi-a-novel-validated-disease-specific-patient-reported-outcome-measure-in-a-24-month-natural-history-study/