Category: Parkinson's Disease: Non-Motor Symptoms
Objective: The current goal is to use pilot data to explain speech changes in patients with Parkinson’s disease (PD) who have Deep Brain Stimulation (DBS) in the subthalamic nucleus.
Background: Approximately 90% of people with PD develop dysarthria characterized by reduced articulatory, respiratory, and phonatory precision resulting in slow, effortful, slurred speech [1,2]. An increasingly common treatment for motor symptoms in refractory PD is surgical implantation of electrodes supplying DBS in the subthalamic nucleus. However, effects on dysarthria severity are variable [3,4]. It is known that PD is associated with reduced vowel space relative to controls [5]. However, the few studies documenting changes in vowel space with stimulation conflict, showing both a relative expansion [6,7] and reduction [8] in vowel space.
Method: Patients with PD and bilateral implantation of DBS within five years produced speech in conditions with DBS on and off. Tasks included production of sustained vowels /a/, /i/, /æ/, and /u/ for three seconds at a habitual pitch and loudness and sentences from the Speech Intelligibility Test [9]. As measures of working vowel space, we calculated vowel space area [10], plus the more sensitive vowel articulation index [11]. We quantified intelligibility using average sentence ratings along a visual analog scale from three naïve raters [12,13].
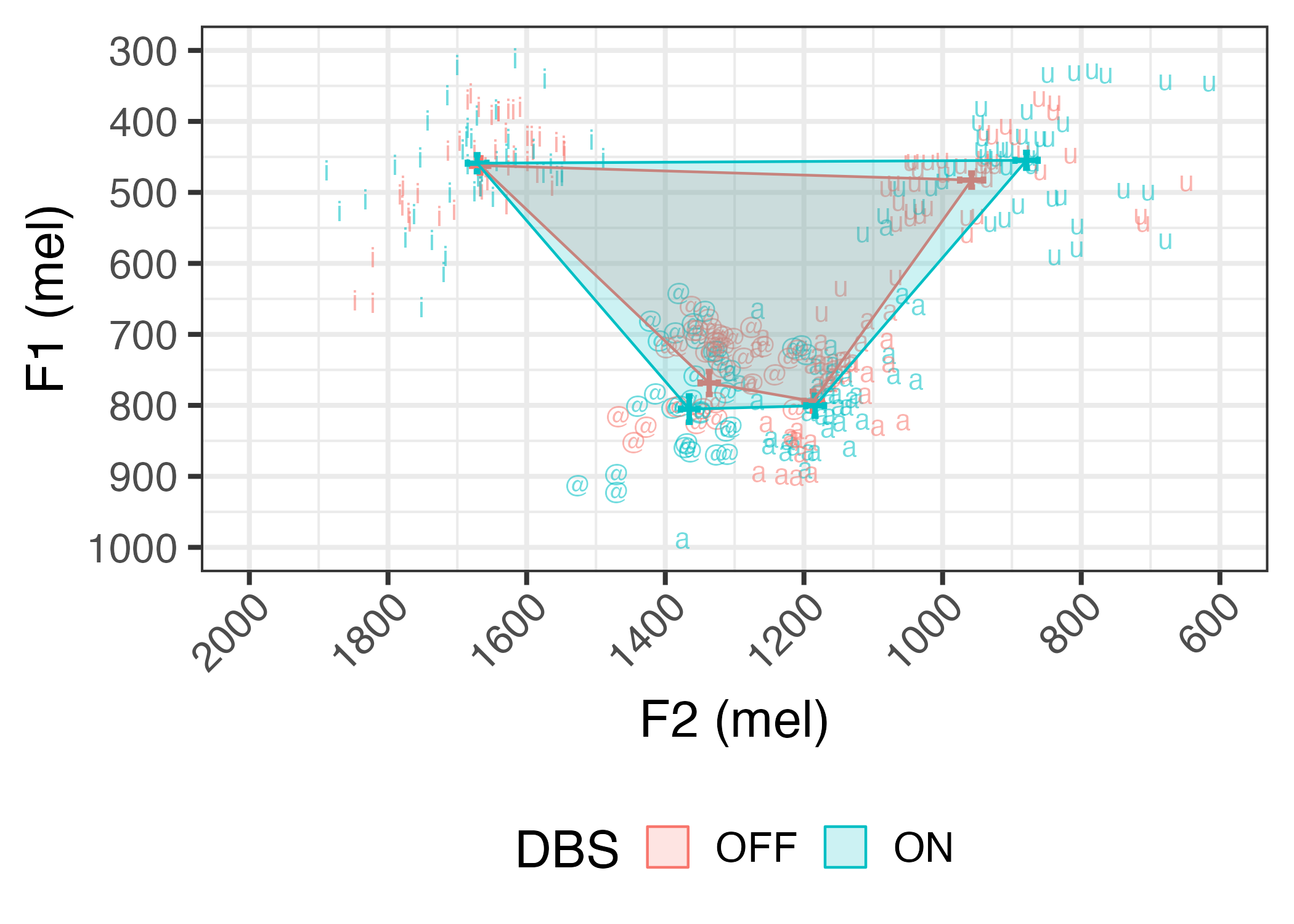
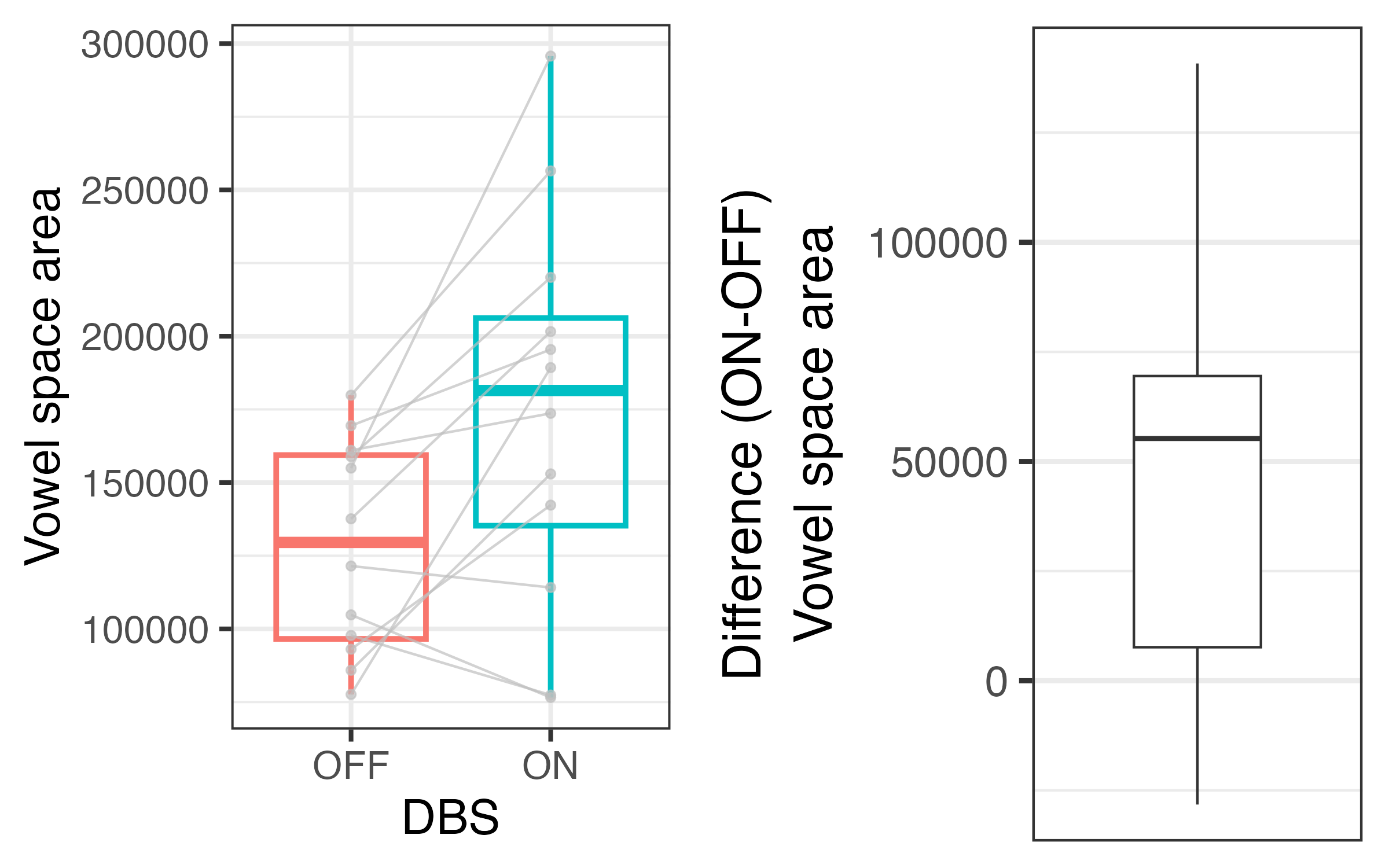
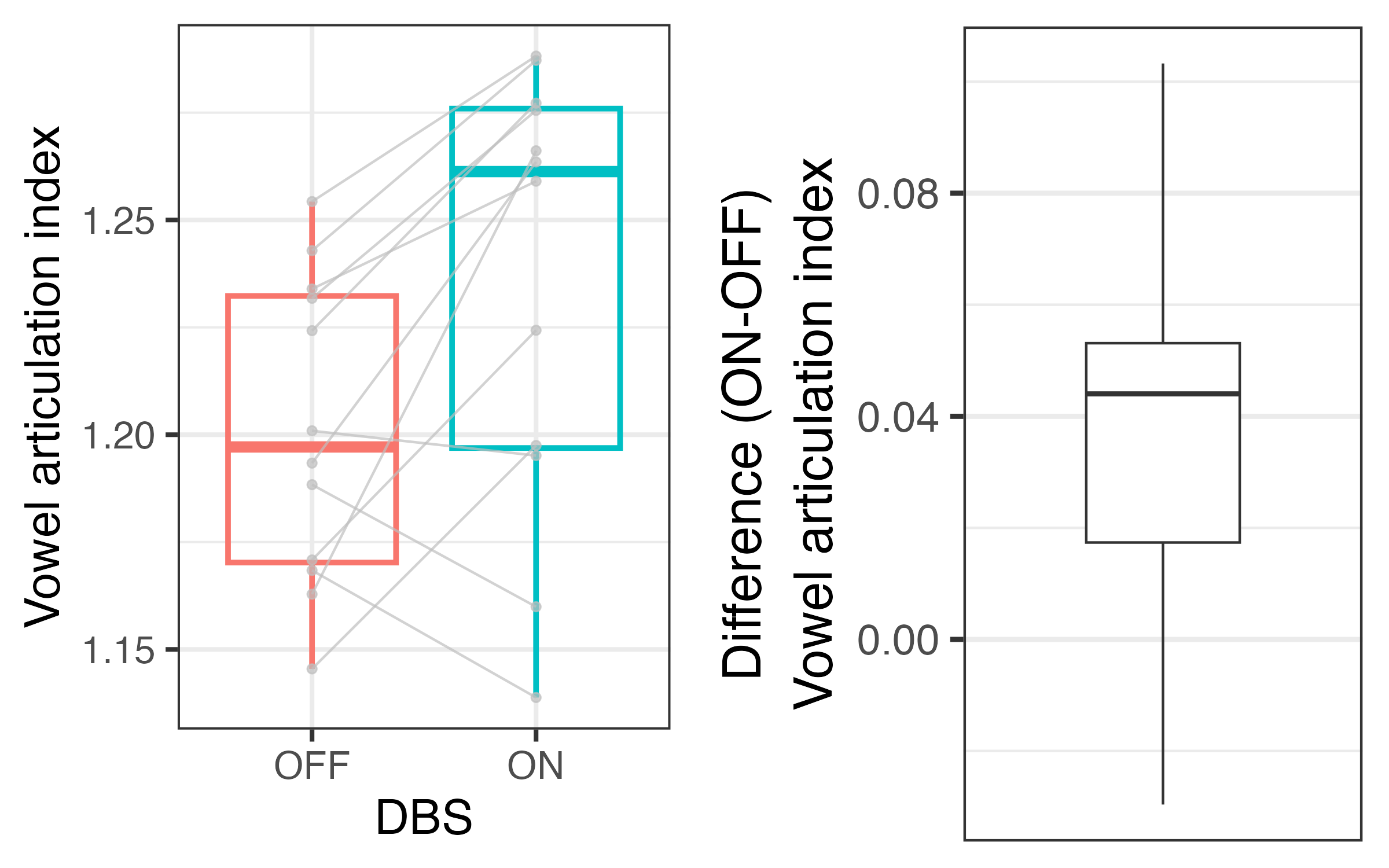
Results: Preliminary inspection of data from thirteen pilot patients suggests that average vowel quadrilaterals are larger with DBS on [figure1], reflected in increased articulatory vowel space [figure2] and vowel articulation index [figure3]. We first correlated vowel space with intelligibility ratings. We then used linear mixed effects regression to predict change in speech from DBS status while controlling for total electrical energy delivered and levodopa equivalent dose, and the patient factors disease duration and severity, time since surgery, and cognition.
Conclusion: Based on preliminary findings, it appears that DBS-STN positively impacts speech in a majority of patients by increasing articulatory vowel space; however, vowel space decreases for a few exceptional patients. We plan further evaluation in twenty patients in hopes of prospectively identifying the few patients who are at greater risk for speech decline with stimulation. This pursuit will inform personalized recommendations for surgical care and/or therapy addressing anticipated speech challenges.
Average vowel space with DBS on and off (n = 13)
Vowel space area in both DBS conditions
Vowel articulation index in both DBS conditions
References: [1] J. R. Duffy, Motor Speech Disorders: Substrates, Differential Diagnosis, and Management, 4th ed. St. Louis, Missouri: Elsevier, 2020.
[2] F. L. Darley, A. E. Aronson, and J. R. Brown, “Differential diagnostic patterns of dysarthria,” J. Speech Hear. Res., vol. 12, no. 2, pp. 246-269, 1969.
[3] S. Skodda, W. Grönheit, U. Schlegel, M. Südmeyer, A. Schnitzler, and L. Wojtecki, “Effect of subthalamic stimulation on voice and speech in Parkinson’s disease: for the better or worse?,” Front. Neurol., vol. 4, p. 218, 2014.
[4] E. Tripoliti et al., “Predictive factors of speech intelligibility following subthalamic nucleus stimulation in consecutive patients with Parkinson’s disease,” Mov. Disord., vol. 29, no. 4, pp. 532-538, 2014.
[5] S. Skodda, W. Visser, and U. Schlegel, “Vowel articulation in Parkinson’s disease,” J. Voice, vol. 25, no. 4, pp. 467-472, 2011.
[6] V. Martel-Sauvageau, J. Macoir, M. Langlois, M. Prud’Homme, L. Cantin, and J.-P. Roy, “Changes in vowel articulation with subthalamic nucleus deep brain stimulation in dysarthric speakers with Parkinson’s disease,” Parkinson’s Disease, vol. 2014, no. 487035, pp. 1-9, 2014.
[7] Y. Tanaka et al., “Articulation features of Parkinson’s disease patients with subthalamic nucleus deep brain stimulation,” J. Parkinsons Dis., vol. 6, no. 4, pp. 811-819, 2016.
[8] J. J. Sidtis, A. G. Alken, M. Tagliati, R. Alterman, and D. Van Lancker Sidtis, “Subthalamic stimulation reduces vowel space at the initiation of sustained production: Implications for articulatory motor control in Parkinson’s disease,” J. Parkinsons Dis., vol. 6, no. 2, pp. 361-370, 2016.
[9] K. Yorkston, D. Beukelman, and R. Tice, Sentence Intelligibility Test. Lincoln, NE: Tice Technologies, 1996.
[10] J. Lam and K. Tjaden, “Clear speech variants: An acoustic study in Parkinson’s disease,” J. Speech Lang. Hear. Res., vol. 59, no. 4, pp. 631-646, 2016.
[11] T. Knowles, S. Adams, A. Abeyesekera, C. Mancinelli, G. Gilmore, and M. Jog, “Deep brain stimulation of the subthalamic nucleus parameter optimization for vowel acoustics and speech intelligibility in Parkinson’s disease,” J. Speech Lang. Hear. Res., vol. 61, no. 3, pp. 510-524, 2018.
[12] D. Abur, N. M. Enos, and C. E. Stepp, “Visual analog scale ratings and orthographic transcription measures of sentence intelligibility in Parkinson’s disease with variable listener exposure,” Am. J. Speech Lang. Pathol., vol. 28, no. 3, pp. 1222-1232, 2019.
[13] K. L. Stipancic, K. Tjaden, and G. Wilding, “Comparison of intelligibility measures for adults with Parkinson’s disease, adults with multiple sclerosis, and healthy controls,” J. Speech Lang. Hear. Res., vol. 59, no. 2, pp. 230-238, 2016.
To cite this abstract in AMA style:
H. Kabakoff, A. Mogilner, M. Pourfar, A. Flinker. Examining the Effects of Deep Brain Stimulation on Speech in Parkinson’s Disease [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/examining-the-effects-of-deep-brain-stimulation-on-speech-in-parkinsons-disease/. Accessed December 19, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/examining-the-effects-of-deep-brain-stimulation-on-speech-in-parkinsons-disease/