Objective: To apply biological definition and Neuronal α-Synuclein Disease Integrated Staging System (NSD-ISS) to the PPMI baseline and longitudinal dataset.
Background: The NSD-ISS Working Group developed a data-driven approach to: 1) determine a biologic definition for disease; and 2) establish a framework for a disease staging platform.
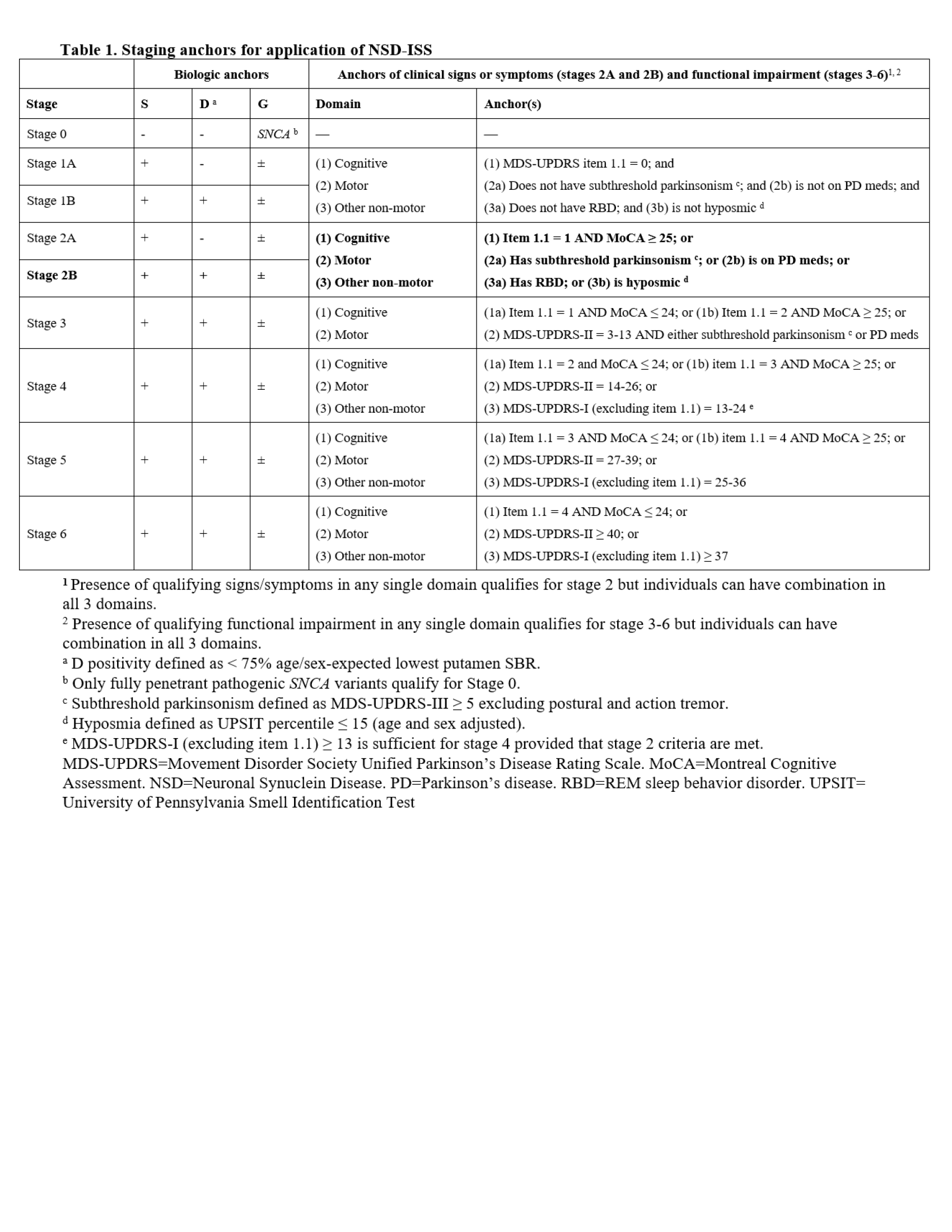
Method: We applied data-driven staging criteria to the PPMI cohort at baseline and longitudinally. Staging anchors are presented in Table 1. This analysis included only PPMI participants who had biomarker data, had NSD and were enrolled by 2020. Biomarker anchors included synuclein (S) assessed by cerebrospinal fluid (CSF) alpha-synuclein seed amplification assay (n-asyn SAA), dopamine (D) assessed by dopamine transporter imaging (DaTscan), and genetic status (G).
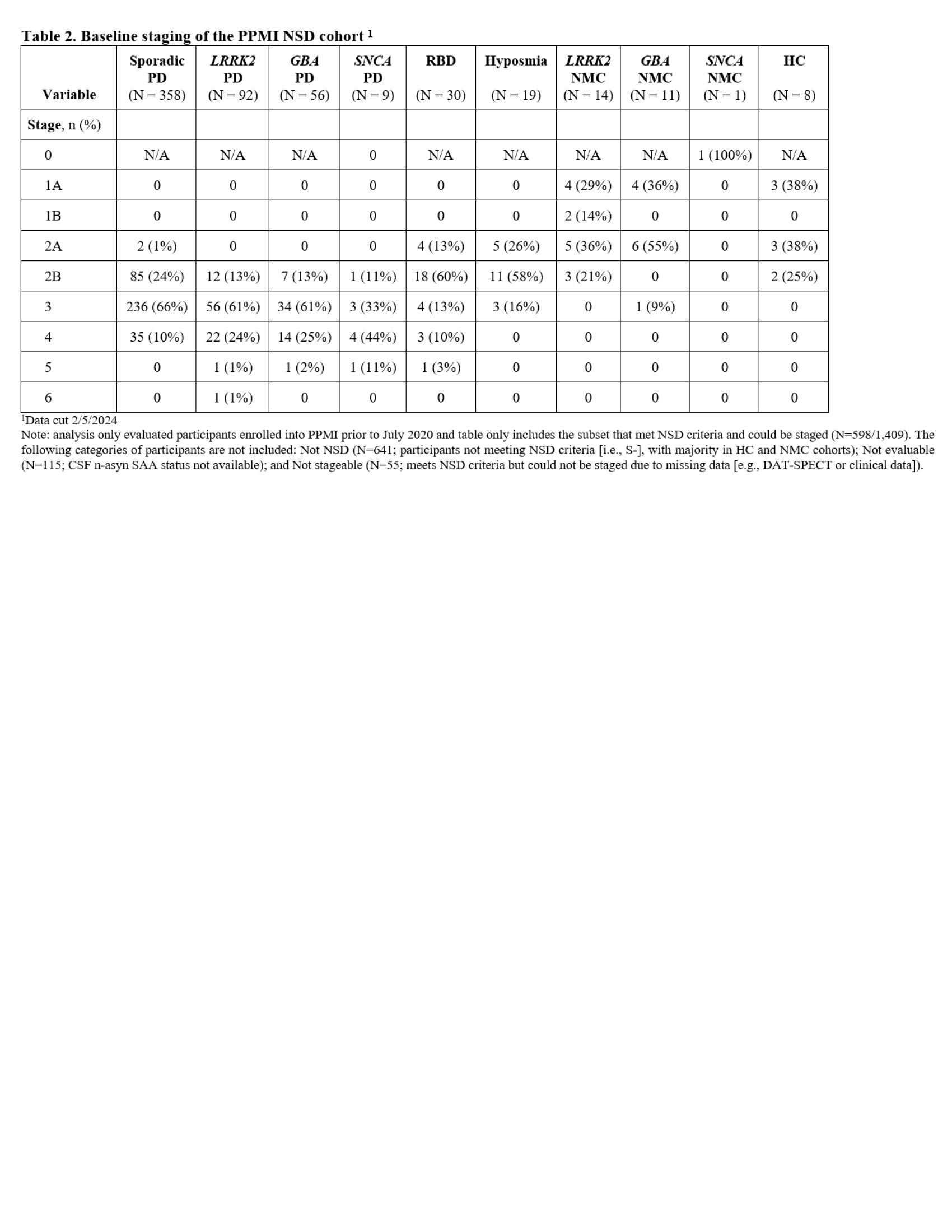
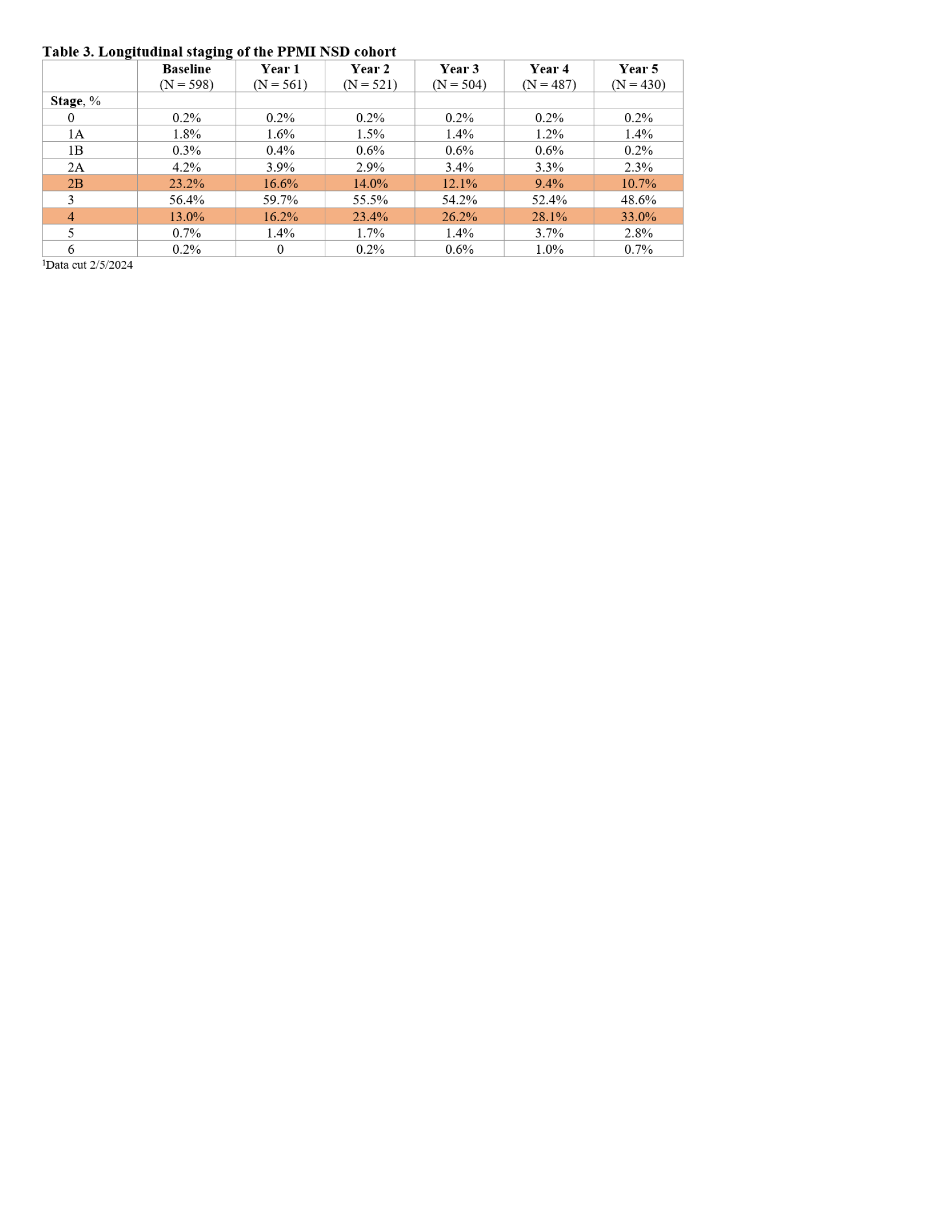
Results: The analysis included 598 NSD participants. Table 2 presents baseline staging based on the recruitment cohorts and subgroups. At baseline, majority of PD participants were in stage 3, majority of prodromal were in stage 2B and non-manifest LRRK2 and GBA carriers were in stages 1A/2A. Table 3 presents distribution of staging in all NSD participants ( including all recruitment cohorts) at years 0-5. There was longitudinal reduction of proportion of individuals in stage 2B and increase in stage 4. Proportion in stage 3 remained stable as reflection of transition from stage 2B and from stage 3 to 4. Time to transition from stage 2B to Stage 3 presented by recruitment cohort (PD versus prodromal) is summarized in Figure 1. 60% of stage 2B PD participants and 30% of prodromals progressed to stage 3 by year 2.
Conclusion: We present the first longitudinal data-driven application of the NSD-ISS, highlighting the heterogeneity of the biological stages in a phenotypically homogeneous cohort and time dependent change in staging across the clinical phenotypes. Our data support validity and utility of the NSD-ISS for stage dependent recruitment into clinical trials specifically targeting stage 2B (currently referred to as prodromal) and stage 3 participants. More data will be presented on the timelines for longitudinal change in stages. Further validation of the anchors in longitudinal cohorts is necessary.
Table 2
Table 1
Table 3
To cite this abstract in AMA style:
M. Brumm, T. Simuni, C. Gochanour, L. Chahine, K. Poston, S. Chowdhury, C. Coffey, T. Dam, P. Dibiaso, T. Foroud, M. Fraiser, K. Kieburtz, C. Kopil, K. Merchant, B. Mollenhauer, G. Pagano, J. Seibyl, T. Sherer, D. Stephenson, C. Tanner, E. Tolosa, D. Weintraub, Y. Xiao, A. Siderowf, B. Dunn, K. Marek. Baseline and Longitudinal NSD-ISS Staging of the PPMI Cohort [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/baseline-and-longitudinal-nsd-iss-staging-of-the-ppmi-cohort/. Accessed April 1, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/baseline-and-longitudinal-nsd-iss-staging-of-the-ppmi-cohort/